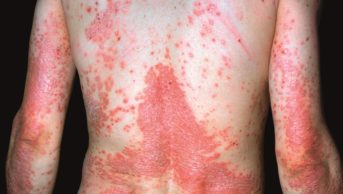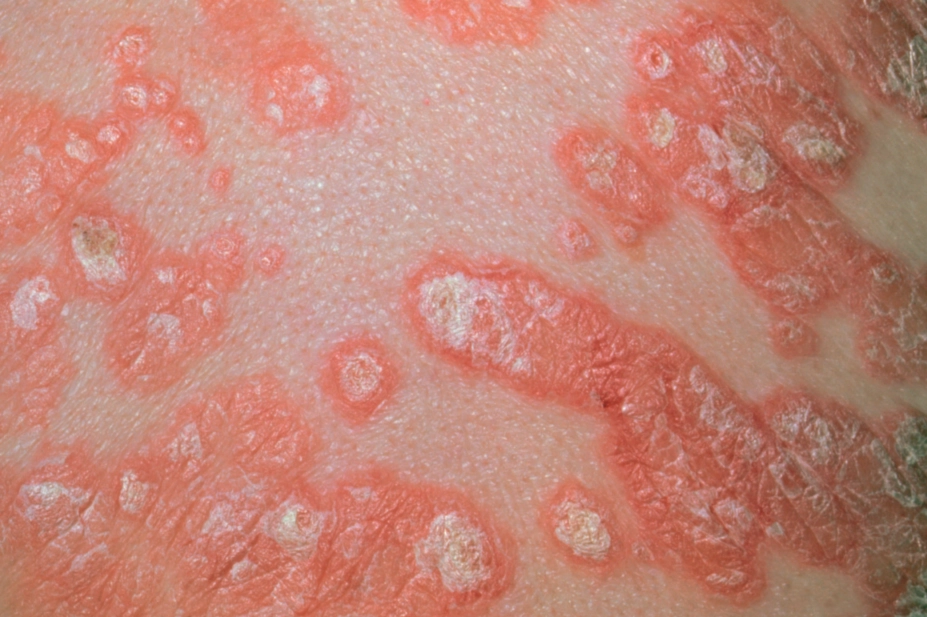
Dr P Marazzi / Science Photo Library
Traditionally, psoriasis was considered only as a skin problem; however, it is now best described as a complex, multifactorial and inflammatory disease[1]
. Psoriasis is thought to affect between 2% and 4% of the population in Westernised countries[2]
and although not life-threatening, it is associated with a significant impairment of quality of life, affecting work, family and sexual relations, as well as physical and psychological wellbeing[3],[4]
. Moreover, the visible nature of the condition has been reported by patients as one of the most difficult aspects[5]
.
Both sexes are affected equally and, for the majority of patients (75%), psoriasis first presents between the ages of 15 years and 25 years, with the remainder of those affected experiencing symptoms between the ages of 55 years and 60 years[6]
.
There are several different forms of psoriasis (see Table 1 and Photoguide 1), but the most common — representing up to 90% of all cases — is chronic plaque psoriasis[7]. This article will focus on the management of adult patients with plaque psoriasis in primary care, but will not discuss the use of specials — covered in publications from the British Association of Dermatologists— or a discussion of treatments provided to patients in secondary care.
| Type of psoriasis | Clinical appearance | Treatment |
|---|---|---|
| Guttate psoriasis | Typically presents as small (1mm–10mm) oval, scaly, red-to-pink plaques over the whole of the body. More common in children and young people, but can occur in adults and is often preceded by a streptococcal throat infection | Although the condition can clear without treatment, ultraviolet (UV) therapy is often used two to three times a week for up to eight weeks |
| Pustular psoriasis | Characterised by white pustules surrounded by inflamed skin. Often seen in adults; can be localised (hands/palms) or may cover most of the body (generalised pustular psoriasis). Patients develop inflamed skin and within hours develop pustules which can coalesce to form lakes of pus | Generalised pustular psoriasis is a medical emergency and death can occur through cardiorespiratory failure. Secondary management involves prevention of fluid loss, stabilising body temperature and correcting electrolyte balance |
| Inverse or flexural psoriasis | Affects intertriginous regions of the body (axilla, natal cleft, genital region). Typical appearance can be described as shiny red, well-defined lesions with no adherent scale | Mild potency topical steroids often in conjunction with an anti-fungal agent, because of the potential for co-existant fungal infections. Vitamin D analogues such as calcitriol can also be used. However, patch testing a small area of skin first is useful because of the potential for irritation |
| Palmoplantar pustular psoriasis | Presents as a series of sterile pustules occuring on the hands (palms) and soles of the feet | A range of treatment options have been used including topical steroids, coal tar, acitretin and phototherapy |
| Erythrodermic psoriasis | A generalised redness and shedding of the skin, can be described as looking like burnt skin. The condition can occur acutely or develop over a few days or even weeks | A potentially life-threatening condition and requires hospitalisation. Treatment involves systemic psoriatic agent, combined with emollients, topical steroids and correction of fluid imbalances |
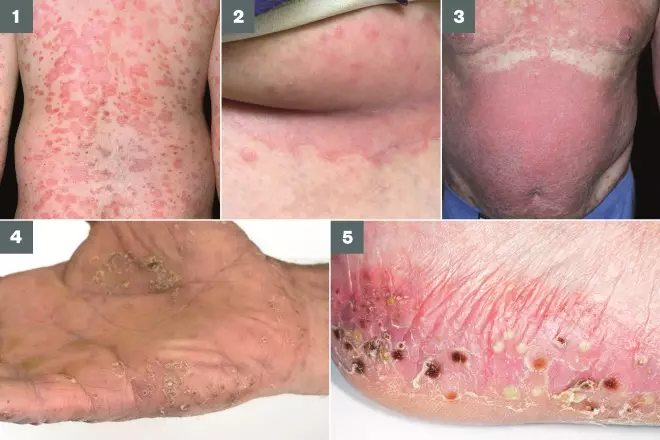
Photoguide 1: Different types of psoriasis
Source: Science Photo Library
1. Guttate psoriasis: Small (1–10mm) oval, scaly, red-to-pink plaques over the whole of the body. More common in children and young people, but can occur in adults and is often preceded by a streptococcal throat infection.
2. Flexular psoriasis below the breast: Shiny red, well-defined lesions with no adherent scale, affects intertriginous regions of the body.
3. Erythrodermic psoriasis: Generalised redness and shedding of the skin, can be described as looking like burnt skin.
4. Palmoplantar psoriasis: Series of sterile pustules occuring on the hands (palms) and soles of the feet.
5. Pustular psoriasis: Characterised by white pustules surrounded by inflamed skin. Often seen in adults; can be localised (hands/palms) or may cover most of the body (generalised pustular psoriasis). Patients develop inflamed skin and, within hours, pustules can coalesce to form lakes of pus
Pathophysiology
The precise cause of psoriasis remains unclear but it is likely that a combination of genetic, environmental and immunological factors is responsible. Prior to the 1980s, it was believed that psoriasis was solely a result of keratinocyte dysregulation, leading to hyper proliferation of these cells in the epidermis. Later work suggested that psoriasis was a T cell-mediated disease and the important role of the pro-inflammatory cytokine, tumour necrosis factor alpha (TNF-α), in psoriasis was illustrated in a study using the anti-TNF-α agent, etanercept, in patients with psoriatic arthritis. Of 19 patients in the etanercept group who had psoriasis, 5 patients achieved a significant improvement in their psoriasis, compared with none in the placebo group[8]
. Subsequent work has shown that other interleukins (IL), such as IL23 and IL17A, have an important role in the disease, although exactly how these interact to cause psoriasis has not yet been explained[9]
.
Diagnosis
Symptoms
Clinically, plaque psoriasis is characterised by well-defined erythematous, silvery-white hyperkeratotic scaling plaques that occur on extensor surfaces of the body (e.g. elbows, knees and the lower back), but psoriasis also affects the scalp (see Photoguide 2). Many patients also experience changes to their nails, including abnormal nail-plate growth leading to characteristic pitting, a build-up of keratinous material underneath the nail (subungual hyperkeratosis) and onycholysis (separation of the nail from its bed) (see Photoguide 2).
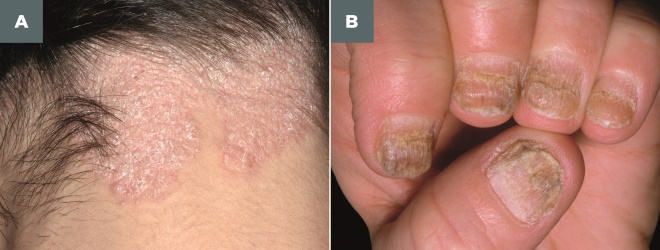
Photoguide 2: Psoriasis of the scalp and fingernails
Source: Science Photo Library
A. Scalp psoriasis: Well-defined erythematous, silvery-white hyperkeratotic scaling plaques.
B. Fingernail psoriasis: Abnormal nail-plate growth leading to characteristic pitting, a build-up of keratinous material underneath the nail (subungual hyperkeratosis) and onycholysis (separation of the nail from its bed)
Psoriasis does occur, although less commonly, in children, where it can be misdiagnosed as atopic eczema, since the scaling is less prominent and lesions often affect the face.
Common co-morbidities and risk factors
Several lifestyle factors appear to be associated with psoriasis. An Italian case–control study found that cigarette smoking, body mass index and stress all appeared to be independently correlated with psoriasis[10]
. Other studies have shown an association between psoriasis and obesity[11]
, alcohol consumption[12]
and physical inactivitiy[13]
. Moreover, there is evidence to suggest that lifestyle modification, such as weight loss[14]
and increased physical activity[15]
, appear to reduce disease severity. Some evidence indicates a potential increased risk of cardiovascular disease given a higher incidence of metabolic syndrome in patients with psoriasis[16]
. However, a review of 14 cohort studies found that while there is a greater risk of cardiovascular disease, this was only significant for patients with severe disease (defined as those requiring systemic therapy or hospital admission)[17]
.
One of the most prevalent co-morbidities is psoriatic arthritis (PsA), a chronic inflammatory, degenerative condition associated with progressive joint disease[18],[19]
. In this instance, early detection may help to halt further development. Screening for PsA can be achieved using the Psoriasis Epidemiological Screening Tool (PEST), which is quick and easy for patients to complete and has a sensitivity of 97% and a specificity of 79%[20]
.
Formal diagnosis of PsA requires assessment by a rheumatologist and, according to the National Institute of Health and Care Excellence (NICE), the health technology assessment body, up to 30% of people with psoriasis have PsA[21]
. NICE guidance recommends that healthcare professionals offer an annual assessment for PsA to people with any type of psoriasis and that if PsA is suspected, the patient should be referred to a rheumatologist for assessment and advice about planning their care[22]
.
In addition, owing to the very visible nature of the disease, patients often experience stress and anxiety, which can lead to depression in around 28% to 67% of patients[23]
.
Assessing the disease severity
The diagnosis of plaque psoriasis is based on clinical symptoms and a consideration of a family history of the disease. By contrast, there is no universally accepted definition of disease severity. According to the National Psoriasis Foundation in the United States, severity can be defined in terms of the total body surface area (BSA) affected[24]
. The surface area of a single hand (i.e. flat palm and five fingers) of the affected person, represents around 1% of the individual’s total skin. Based on this measure, mild psoriasis affects less than 3% BSA, moderate between 3% to 10% BSA, and severe more than 10% BSA[24]
.
One of the most comprehensive measures of disease severity employed in clinical studies is the psoriasis area and severity index (PASI). This assesses disease severity in four separate body regions and the values summed so that PASI scores range from 0–72, with higher scores reflecting greater disease severity[25]
. Several useful calculators are available to help calculate PASI scores (see ‘useful resources’ box below).
NICE uses the PASI tool as qualifying criteria for treatment, such that scores less than 10 represent mild disease, greater than 10 but less than 20 represent moderate disease and anyone scoring over 20 has severe disease[22]
. In addition, a physician or patient global assessment of disease severity rated from clear to severe is also sometimes reported in trials.
While PASI scores represent a more comprehensive measure of disease severity compared with BSA, neither tool considers the impact on quality of life. In recent years, severity has been re-defined by a European consensus group, incorporating BSA, PASI and quality of life, measured using the dermatology quality of life index (DLQI). Severity is dichotomised into either mild or moderate-to-severe. Mild psoriasis is defined when all three measures have a value less than ten and moderate-to-severe where all three measures are greater than ten[26]
.
Management of psoriasis in primary care
Fortunately, the majority of patients with plaque psoriasis (around 80%) have mild-to-moderate disease that is amenable to treatment in primary care with topical therapies[27]
. Patients with more widespread disease would normally be referred and managed in secondary care with a greater range of treatments including ultraviolet (UV) therapy, disease-modifying anti-rheumatic drugs (DMARDs), or even biologic agents in those with the most severe disease.
As there is currently no cure for psoriasis and the condition follows a relapsing-remitting pattern, it is important to assess the relative effectiveness of treatments to ensure that patients achieve a satisfactory response and remain in remission for as long as possible. There are a number of topical therapeutic agents available and a summary of these agents, their onset of action and main adverse effects is provided in Table 2.
The most recent systematic reviews of topical therapies for psoriasis are a Cochrane review with data searches up to August 2012[28]
and the review by NICE that was conducted over a similar time period (up to March 2012)[21]
, which has led to treatment recommendations outlined in this article.
| Drug class | Mode of action/use | Onset of clinical effect (weeks) | Most common adverse effects |
|---|---|---|---|
| Adapted from Nast et al. 2012[29] | |||
| Topical steroids | Mainly anti-inflammatory effects. Potent steroids are recommended for trunk and limbs, whereas mild potency agents are reserved for use on the face and in flexures. Applied once or twice daily using the fingertip unit as a guide (this covers an area of approximately a single hand) and is continued for up to four weeks. When applying to a plaque, use a circular motion to cover evenly, leaving a visible thin layer on the plaque | 1–2 | Burning or stinging at the site of application. May worsen existing conditions such as acne or rosacea |
| Vitamin D analogues | Unclear, but thought to involve suppression of pro-inflammatory cytokines and inhibition of keratinocyte proliferation and increased differentiation of keratinocytes. Generally used on trunk or limbs, although can also be used in flexures, but generally too irritating for the face. Apply a thin layer to plaques, once or twice daily for up to eight weeks | 1–2 | Skin irritation such as erythema, pruritus and burning |
| Dithranol | Suppression of cell proliferation of keratinocytes, proinflammatory cytokines. Normally used as “short-contact regime”, starting at a low strength (0.1%), applying a small amount to the plaque until fully absorbed. If tolerated the strength can be titrated up over four weeks to the highest strength tolerated. The treatment is applied for between 30 minutes and 60 minutes, then washed off. Improvement can take up to six weeks. However, it can be left on overnight if necessary | 2–3 | Burning and eythema in the skin. In practice, reducing the irritancy to normal skin can be achieved by covering the surrounding skin with white soft paraffin |
| Coal tar | Still unclear but thought to be anti-proliferative/anti-inflammatory. Applied as a thin layer to plaques two to three times a day | 4–8 | Minimal but patients may experience skin irritation and even photosensitivity. Many patients dislike the odour of coal tar |
| Tazarotene | Affects epidermal proliferation and differentiation by acting on retinoid receptors in the skin. Applied as a thin layer in the evening for up to three months | 1–2 | Pruritus, burning, erythema at the site of application and irritation |
Treatments
Emollients
Although generally not covered in clinical guidelines, emollients potentially have an important adjunctive role in psoriasis, helping to soften and hydrate the stratum corneum, enhance desquamation (shedding) of hyperkeratotic skin and providing relief of pruritus. There is also a suggestion that emollients might enhance the penetration of other topical psoriasis treatments. Despite their widespread use in psoriasis and a British National Formulary (BNF) recommendation that these agents might be the only necessary treatment in mild psoriasis, there is a lack of robust data on the efficacy of emollients in psoriasis, although some evidence suggests that products containing urea or salicylic acid may be of benefit[30]
.
Emollients should be used daily to help reduce dry, rough, flaky and scaly skin. They can be applied around 30 minutes before any active treatments and they should be used as a soap substitute in the bath or shower. Patients should be advised that after showering or bathing they should moisturise their whole body, as well as moisturising any dry or itchy areas of skin or psoriasis during the day. Patients should avoid scratching or picking psoriasis plaques.
Pharmacists and healthcare professionals should warn patients that some paraffin-based emollients pose a fire risk.
Combination therapy
To date, the only commercial combination topical therapy is calcipotriol (a vitamin D analogue) and the potent topical steroid, betamethasone. The product was originally only available as an ointment but it is now also available as a gel, applied either alone or via an applicator device and a foam formulation. Several systematic reviews have assessed the effectiveness of the combination therapy, with the most recent including data up to April 2015[31]
. This systematic review concluded that the combination therapy was more effective than either of the components used alone. This conclusion was also mirrored in the Cochrane review[28]
. However, by contrast, NICE recommended that the two components (calcipotriol and betamethasone) should be used together, albeit separately, one in the morning and one in the evening[21]
(because of the high cost of the combination product), but members of the NICE psoriasis guideline development group appeared to accept in a subsequent publication that once daily application of the combination product was as effective as using either agent separately[32]
.
Guidelines
In 2012, NICE issued a guideline on the assessment and management of plaque psoriasis. However, other guidelines produced in the UK are inconsistent with the advice from NICE, which is potentially confusing for healthcare professionals.
As an initial therapy, NICE recommended that practitioners offer a potent corticosteroid applied once daily, plus vitamin D or a vitamin D analogue applied once daily (applied separately, one in the morning and the other in the evening) for up to four weeks as initial treatment for adults with trunk or limb psoriasis[22]
.

In addition, given the potent risks of using potent topical steroids long-term, the guideline advises that patients be reviewed after four weeks of treatment. If the combination appears to be effective, then the topical steroid should not be used at the same site for longer than eight weeks and treatment can be continued with the vitamin D analogue, allowing for a four-week “steroid free” interval.
Guidance from the Scottish Intercollegiate Guidelines Network (SIGN) is slightly different and states that, for initial treatment, short-term intermittent use of a potent topical corticosteroid or a combined potent corticosteroid plus calcipotriol ointment is recommended to gain rapid improvement in plaque psoriasis[33]
.
The Primary Care Dermatology Society advice aligns more with the SIGN rather than NICE, and states that many GPs and GPs with a special interest use calcipotriol and betamethasone combination products first-line to encourage a rapid improvement and hence adherence in chronic plaque psoriasis. Advice therefore differs from the NICE psoriasis guideline which suggests starting with its individual components[34]
.
This latter recommendation has clearly taken route in primary care. The 2016 prescribing data for England and Wales show that 55% of all topical psoriasis treatments issued were for the various formulations of the combination product (e.g. gel, ointment and foam)[35]
.
Scalp psoriasis
Involvement of the scalp occurs in up to 79% of patients with trunk or limb psoriasis and is often the first area to show signs of the disease[36]
. Clinically, scalp lesions are thickened, well demarcated, inflamed, scaling plaques that are often pruritic (see Photoguide 2). There are several treatment options for scalp psoriasis, including a range of over-the-counter shampoos, which contain coal tar and/or salicylic acid, to prescription products based on topical steroids and vitamin D analogues. However, the evidence on the effectiveness of these treatments is limited.
In a recent review of the evidence, involving the analysis of 59 randomised trials with more than 11,500 patients, a Cochrane review concluded that the combination of a potent topical steroid and a vitamin D analogue was more effective than a steroid alone, although the clinical benefit was questionable. Moreover, the authors were unable to assess the efficacy of other topical agents such as tar, salicylic acid or dithranol[37]
.
Given the lack of efficacy data on over-the-counter shampoos containing tar or salicylic acid, it would be prudent for pharmacists to refer patients with scalp psoriasis to their GP unless they have very mild disease, in which case shampoos may be of some benefit.
Treatment adherence
Adherence to topical regimes in psoriasis is generally poor. For example, a study by Storm et al. found that 44% of patients failed to redeem their initial prescription[38]
, while another study by Richards et al. found that 39% were non-compliant with their recommended treatment regime[39]
. A better understanding of the factors associated with adherence may help pharmacists and other healthcare professionals support patients to facilitate improved adherence. However, the evidence from reviews of the literature suggest that adherence is a complex issue. For instance, a review by Devaux et al found that the most frequent factors associated with reduced adherence were low treatment efficacy, the time required for application and reduced cosmetic acceptability of the formulation[40]
. Nevertheless, a subsequent review by Thorneloe et al. concluded that the current evidence makes it difficult to ascertain the most important determinants of adherence[41]
. Finally, a recent review by Belinchon et al. suggested that incorporating patient references into the treatment decision-making process may contribute to improved treatment, satisfaction, adherence and ultimately clinical outcomes, although there was no direct evidence to support this advice[42]
.
A potential problem with promoting greater adherence, as identified in the review by Thorneloe et al., is that as patients become expert at managing their condition, they may alter the medication regime to suit themselves so that while disease severity may not be optimally improved, the strategy may improve their quality of life[41]
.
How pharmacists can help patients with psoriasis
Qualitative studies in those patients affected by psoriasis have revealed an often erratic and inconsistent use of topical therapies, a desire for advice on the correct use of topical therapies (which is usually absent in consultations) and a perception that healthcare professionals lack sufficient knowledge and expertise of psoriasis[43],[44],[45]
. Consequently, patients frequently sub-optimally manage their condition and in isolation from healthcare professionals because of the absence of adequate advice on treatments. These studies clearly illustrate the need for more effective community-based information, education and support for patients with psoriasis. There is some evidence to suggest that adjunctive patient education for those with skin problems such as atopic eczema, leads to improvements in disease outcomes[46]
, although it was concluded that educational interventions for patients with psoriasis employed in clinical trials have met with limited success with respect to improving disease severity and quality of life[47]
.
In a recent study exploring the educational impact of pharmacists’ advice to patients with psoriasis, it was found that their advice and support led to significant improvements in patients’ understanding of their condition, disease severity and quality of life[48]
. In the study, pharmacists used a validated tool to assess patients’ knowledge and understanding of their psoriasis and patients’ self-assessed disease severity and quality of life[49]
. Pharmacists held an initial consultation and one follow-up appointment six weeks later, at which point all outcome measures had improved significantly. In follow-up interviews of patients and pharmacists, both parties agreed that the intervention was of value and the pharmacists perceived themselves to be important members of the primary healthcare team caring for those with psoriasis[50]
. However, further work is needed to explore in more detail the potential impact of advice from pharmacists on disease outcomes in those with psoriasis.
Box 1 provides some helpful counselling points pharmacists can pass on to patients with psoriasis.
Box 1: Counselling points pharmacists can pass on to patients with psoriasis
- Find an emollient or range of emollient products for washing and moisturising that you like and suit you, and use them each day;
- Moisturise your entire body (including psoriasis areas) each day using long, smooth, soothing strokes (in the direction of hair growth);
- Carry a small amount of moisturiser with you and apply to dry and itchy plaques during the day;
- Avoid scratching and picking;
- Leave around 20–30 minutes between emollient and psoriasis topical treatments, to aid the effectiveness of each;
- Persevere with topical psoriasis treatments for at least one month — most take around four weeks to begin to work;
- Be aware that the scale will disappear first and the psoriasis may appear red — this is normal and continuing with treatment will result in pink areas, which will gradually fade to normal skin colour;
- If you have scalp psoriasis, de-scale your scalp before applying topical treatments. Remember to part the hair and treat the scalp;
- Work out a good individual routine — emollients should be used daily and psoriasis treatments only when condition is flaring;
- Once the skin becomes flat, active treatments can be stopped, although you should continue with emollients.
Diet and psoriasis
Patients may ask pharmacists about the role of diet in psoriasis and whether there are specific foods that should be eaten or avoided. There is some evidence that consumption of omega-3 fish oils can improve disease severity and that supplementing with oral vitamin D could help, although the evidence from studies is less robust[51]
.
The Royal Pharmaceutical Society, in its report ‘Improving care for people with long term conditions’ , suggests that pharmacists could help patients with long-term conditions and assisting those with psoriasis provides an opportunity to embrace that vision[52]
. Although presently limited in scope, the evidence that does exist offers some insight into how pharmacists could make an important contribution to the care of people with psoriasis.
Useful resources:
UK patient support organisations:
Financial and conflicts of interest disclosure:
The author was a member of the NICE group who developed the psoriasis guidelines and has received research grants and speaker fees from Leo Pharma, Mylan and Galderma. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Habashy J & Robles DT. Psoriasis. emedicine 2017. Available at: https://emedicine.medscape.com/article/1943419-overview (accessed March 2018)
[2] Parisi R, Symmons DPM, Griffiths CEM et al. Global Epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377–385. doi: 10.1038/jid.2012.339
[3] Langley RGB, Krueger GG & Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005;64(Suppl 2):18–23. doi: 10.1136/ard.2004.033217
[4] Kimball AB, Jacobson C, Weiss S et al. The psychosocial burden of psoriasis. Am J Clin Dermatol 2005;6(6):383–392. PMID: 16343026
[5] Uttjek M, Nygren L, Stenberg B et al. Marked by visibility of psoriasis in everyday life. Qual Health Res 2007;17(3):364–372. doi: 10.1177/1049732306297674
[6] Griffiths CEM, Barker JNWN, Chalmers RJG et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007;156:258–262. doi: 10.1111/j.1365-2133.2006.07675.x
[7] Weigle N & McBane S. Psoriasis. Am Fam Physician 2013;87(9):626–633. Available at: https://www.aafp.org/afp/2013/0501/p626.html (accessed March 2018)
[8] Mease PJ, Goffe BS, Metz J et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000;356:385–390. doi: 10.1016/S0140-6736(00)02530-7
[9] Lynde CW, Poulin Y, Vender R et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol 2014;71:141–50. doi: 10.1016/j.jaad.2013.12.036
[10] Naldi L, Chatenoud L, Linder D et al. Cigarette smoking, Body mass index and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol 2005;125:61–67. doi: 10.1111/j.0022-202X.2005.23681.x
[11] Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes 2012;2:e54. doi: 10.1038/nutd.2012.26
[12] Kirby B, Richards HL, Mason DL et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol 2007;158(1):138–140. doi: 10.1111/j.1365-2133.2007.08299.x
[13] Frankel HC, Han J, Li T et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol 2012;148(8):918–924. doi: 10.1001/archdermatol.2012.943
[14] Upala S & Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes 2015;39(8):1197–2002. doi: 10.1038/ijo.2015.64
[15] Naldi L, Conti A, Cazzaniga S et al. Diet and physical exercise in psoriasis: a randomised controlled trial. Br J Dermatol 2014:170(3):634–642. doi: 10.1111/bjd.12735
[16] Gisondi P, Tessari G, Conti A et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol 2007;157(1):68–73. doi: 10.1111/j.1365-2133.2007.07986.x
[17] Samarasekera EJ, Neilson JM, Warren RB et al. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol 2013;133:2340–2346. doi: 10.1038/jid.2013.149
[18] Kane D, Stafford L, Bresnihan B et al. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinical experience. Rheumatology (Oxford) 2003;42(12):1460–1468. doi: 10.1093/rheumatology/keg384
[19] McHugh NJ, Balachrishan C & Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology (Oxford) 2003;42:778–783. doi: 10.1093/rheumatology/keg217
[20] Machado PM & Raychaudhuri SP. Disease activity measurements and monitoring in psoriatic arthritis and axial spondyloarthritis. Best Pract Res Clin Rheumatol 2014;28(5):711–728. doi: 10.1016/j.berh.2014.10.004
[21] National Institute for Health and Care Excellence (NICE). Etanercept, infliximaba and adalimumab for the treatment of psoriatic arthritis. Available at: https://www.nice.org.uk/guidance/ta199/chapter/2-Clinical-need-and-practice (accessed March 2018)
[22] National Institute for Health and Care Excellence (NICE). Psoriasis: assessment and management. Available at: https://www.nice.org.uk/guidance/cg153/chapter/1-Guidance#principles-of-care (accessed March 2018)
[23] Lakshmy S, Balasundaram S, Sarkar S et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med 2015;37(4):434–440. doi: 10.4103/0253-7176.168587
[24] National Psoriasis Foundation. About psoriasis. Available at: https://www.psoriasis.org/about-psoriasis (accessed March 2018)
[25] Ashcroft DM, Li Wan A, Williams HC et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol 1999;141:185–191. PMID: 10468786
[26] Mrowietz U, Kragballe K, Spuls RK et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 2011;303(1):1–10. doi: 10.1007/s00403-010-1080-1
[27] Menter A, Koman NJ, Fledman SR et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009;60:643–659. doi: 10.1016/j.jaad.2008.12.032
[28] Mason AR, Mason J, Cork M et al. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev 2013; Issue 3. Art. No.: CD005028. doi: 10.1002/14651858.CD005028.pub3
[29] Nast A, Boehncke WH, Mrowietz U et al. S3 – Guidelines on the treatment of psoriasis vulgaris (English version). Update. J Dtsch Dermatol Ges 2012;10(2):S1–S95. doi: 10.1111/j.1610-0387.2012.07919.x
[30] Jacobi A, Mayer A & Augustine M. Keratolytics and emollients and their role in the therapy of psoriasis: a systematic review. Dermatol Ther (Heidelb) 2015;5:1–18. doi: 10.1007/s13555-015-0068-3
[31] Yan R, Jiang S, Wu Y et al. Topical calcipotriol/betamethasone dipropionate for psoriasis vulgaris: a systematic review. Indian J Dermatol Venereol Leprol 2016;82(2):135–144. doi: 10.4103/0378-6323.175919
[32] Samaraskera EJ, Sawyer L, Wonderling D et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analysis. Br J Dermatol 2013;168:954–967. doi: 10.1111/bjd.12276
[33] Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and management of psoriasis and psoriatic arthritis. Available at: http://sign.ac.uk/guidelines/fulltext/121/index.html (accessed March 2018)
[34] Primary Care Dermatology Society. Chronic plaque psoriasis guideline. Available at: http://www.pcds.org.uk/clinical-guidance/psoriasis-an-overview#management (accessed March 2018)
[35] NHS Digital. Prescription cost analysis, 2016. Available at: https://digital.nhs.uk/catalogue/PUB23631 (accessed March 2018)
[36] Van de Kerkhof PC, de Hoop D, de Korte J et al. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology 1998;197:326–334. doi: 10.1159/000018026
[37] Schlager JG, Rosumeck S, Werner RN et al. Topical treatments for scalp psoriasis. Cochrane Database of systematic reviews 2016, Issue 2. Art. No. CD009687. doi: 10.1111/bjd.14811
[38] Storm A, Andersen ES, Benfeldt E et al. One in three prescriptions are never redeemed: primary non-adherence in an outpatient clinic. J Am Acad Dermatol 2008;59:27–33. doi: 10.1016/j.jaad.2008.03.045
[39] Richards HL, Fortune DG, O’Sullivan TM et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol 1999;41(4):581–583. PMID: 10495380
[40] Devaux S, Castela A, Archier E et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2012;26(S3):61–67. doi: 10.1111/j.1468-3083.2012.04525.x
[41] Thorneloe RJ, Bundy C, Griffiths CEM et al. Adherence to medication in patients with psoriasis: a systematic literature review. Br J Dermatol 2013;168:20–31. doi: 10.1111/bjd.12039
[42] Belinchon I, Rivera R, Blanch C et al. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adherence 2016;10:2357–2367. doi: 10.2147/PPA.S117006
[43] Ersser SJ, Cowdell FC, Latter SM et al. Self-management experiences in adults with mild to moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol 2010;163:1044–1049. doi: 10.1111/j.1365-2133.2010.09916.x
[44] Nelson PA, Chew-Graham CA, Griffiths CE et al. IMPACT Team. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol 2013;168:354–361. doi: 10.1111/j.1365-2133.2012.11217.x
[45] Nelson PA, Barker Z, Griffiths CE et al. IMPACT Team. ‘On the surface’: a qualitative study of GPs’ and patients’ perspectives on psoriasis. BMC Fam Pract 2013;14:158. doi: 10.1186/1471-2296-14-158
[46] de Bes J, Legierse CM, Prinsen CA et al. Patient education in chronic skin diseases: a systematic review. Acta Derm Venereol 2011;91(1):12–17. doi: 10.2340/00015555-1022
[47] Larsen MH, Hagen KB, Krogstad AL et al. Limited evidence of the effects of patient education and self-management interventions in psoriasis patients: a systematic review. Patient Educ Couns 2014;94(2):158–169. doi: 10.1016/j.pec.2013.10.005
[48] Tucker R & Stewart D. The role of community pharmacists in supporting self-management in patients with psoriasis. Int J Pharm Pract 2017;25:140–146. doi: 10.1111/ijpp.12298
[49] Cowdell F, Ersser SJ, Gradwell C et al. The person-centered dermatology self-care index: a tool to measure education and support needs of patients with long-term skin conditions. Arch Dermatol 2012;148(11):1251–1255. doi: 10.1001/archdermatol.2012.1892
[50] Tucker R & Stewart D. Patient and pharmacist perceptions of a pharmacist-led educational intervention for people with psoriasis. Self Care 2016;7(4):18–31. Available at: http://selfcarejournal.com/article/patient-pharmacist-perceptions-pharmacist-led-educational-intervention-people-psoriasis/ (accessed March 2018)
[51] Millsop JW, Bhavnit K, Debbaneh BA et al. Diet and psoriasis: part 3. Role of nutritional supplements. J Am Acad Dermatol 2014;71(3):561–569. doi: 10.1016/j.jaad.2014.03.016
[52] Royal Pharmaceutical Society. Improving care for people with long term conditions. Available at: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Policy/LTC%20-%20England.pdf (accessed March 2018)