
Mclean/shutterstock.com
The concept of producing biological drugs in plants has been around since the 1980s.
“One of the great attractions initially was the idea that you could do things cheaply and at great scale, using some of the methods and distribution networks used in farming,” says George Lomonossoff, a virologist at the John Innes Centre, an independent centre for research and training in plant and microbial science, based in Norwich, UK.
However, it is only now that the technique, known as ‘molecular farming’, has really started to take off.
The first drug on the market to be produced in plant cells, Elelyso (taliglucerase alfa), was approved by the United States Food and Drug Administration (FDA) in 2012. Developed by Israeli biotech company Protalix and Pfizer, it is a treatment for Gaucher disease, a rare genetic disorder affecting up to 1 in 40,000 people worldwide.
Those with the condition are unable to produce the enzyme glucocerebrosidase — which breaks down cellular materials for reuse — leading to symptoms such as fragile bones and an enlarged spleen. The condition is treated by injecting the missing enzyme, and the drug created by Protalix and Pfizer is produced using cells from the humble carrot.
In February 2022, biotech company Medicago announced that it had gained approval in Canada for its two-dose COVID-19 vaccine Covifenz. Phase III results show that the vaccine has similar efficacy rates to several of the other COVID-19 vaccines, but there is a major difference: it is the world’s first plant-based COVID-19 vaccine.
A more efficient alternative
Therapeutic proteins are generally produced using the bacteria Escherichia coli (E. coli), or yeasts, such as Saccharomyces cerevisiae. In cases where the biotherapeutics need to include modifications that are not reproducible in bacteria, Chinese hamster ovary cells (CHO) are often used, owing to their rapid growth and high protein output.
However, all of these approaches require expensive stainless-steel bioreactors and highly controlled, sterile conditions (see Figure). In addition, the proteins they produce vary in their quality and similarity to the human versions they are mimicking. All these factors have led to increased interest in plant-based alternatives, and the discovery that plants might be able to provide a cheaper, more efficient way to produce biological drugs — potentially producing a superior product.
Alisdair Macdonald
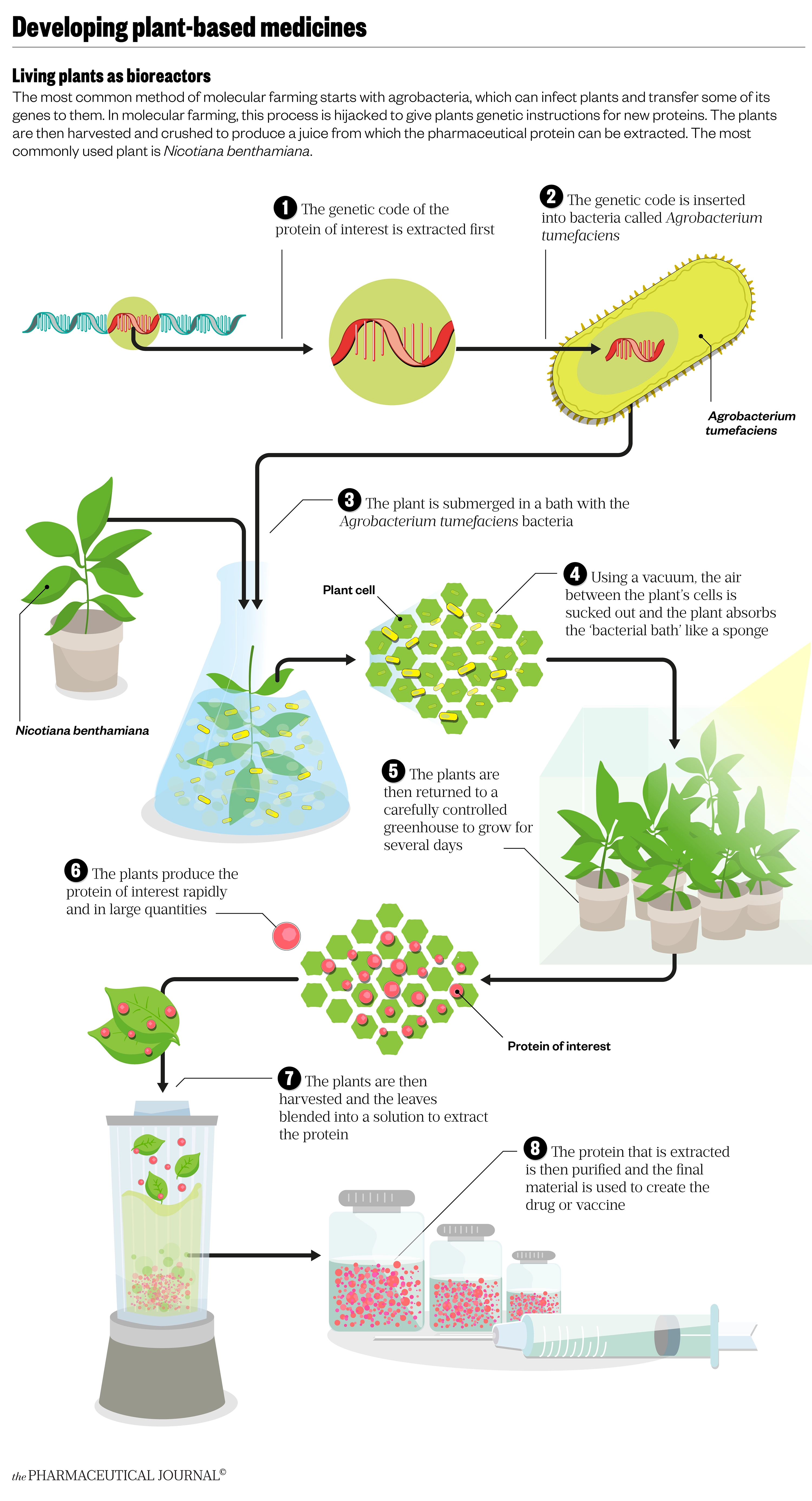
Box: Producing biopharmaceuticals in plants
Since the emergence of the field of molecular farming, several methods for producing biopharmaceuticals in plants have been developed, using both plant cell cultures and whole plants.
Plant cell culture
The first drug on the market, Elelyso (taliglucerase alfa), developed by Protalix and Pfizer, uses a culture of carrot cells, grown in a liquid medium. The cells are grown in disposable 10–800-L bioreactors so that they do not have to be cleaned between cycles, reducing costs dramatically.
The process starts with samples of the plant, from which groups of unorganised cells, called calluses, grow. The cells are then suspended in a liquid culture and provided with the light they need to be able to grow.
The soil bacterium Agrobacterium tumefaciens is then added to the culture. The bacteria are engineered with the gene that codes for the protein that is being made — this code is first inserted into E. coli and then transferred to the agrobacterium.
The bacterium has the ability to easily infect plant cells and, consequently, transfers its DNA containing this gene into the plant cells.
The gene is then expressed in the plant cells in a process known as ‘transient expression’.
Finally, the protein is extracted from the cell culture and purified using similar filtration chromatography and centrifugation steps to the production of other biologics.
Whole plants
One of the most prominent methods currently being developed is plant-based or agro-infusion. Rather than using a cell culture, this approach starts with the whole plant.
The biotech company Medicago began using this method in 2005 because of its capacity to quickly and accurately produce large quantities of the desired protein product.
The method is similar to that developed by Protalix and Pfizer, but the Agrobacterium tumefaciens culture, engineered with the protein of interest, is infused into plant leaves. This can be done by injecting the leaves directly or, on a larger scale, by placing the plant and culture in a vacuum tank that sucks out air, replacing it with the culture medium; much like saturating a sponge in a bucket of water.
The plants are then removed from the culture to dry and, after about a week, the leaves are harvested so that the protein can be extracted and purified.
The early ambitions of wholesale ‘farming’ of drugs have not come to fruition, mainly owing to the need to keep such plants in contained environments to prevent external contamination, explains Lomonossoff. But there is still a hope that growing at scale can be achieved using greenhouses.
The other advantage is safety; one of the problems with mammalian cell culture is the risk of contamination with viruses.
“With plant-based material, that’s much less of an issue, in that any disease a plant might get would not infect humans — there are no examples of crossover,” says Lomonossoff.
For this reason, the FDA has been surprisingly relaxed about the conditions of growth, says Yuri Gleba, founder of Munich-based company, Nomad Bioscience.
“It agreed that the growth of the plant has to be under controlled conditions, but not Good Manufacturing Practice (GMP) conditions … GMP starts when you take the leaf and/or the whole plant and start grinding it.” GMP describes the minimum standard that a medicines manufacturer must meet in their production processes and will require inspections to verify compliance, which increases costs.
Although the initial promise of lower production costs have not been fulfilled so far, the method itself has certainly proven to be speedy. In 2012, Medicago manufactured 10 million doses of an influenza vaccine candidate within one month, and it has been similarly quick in developing its COVID-19 vaccine.
From the moment we had the sequence on our computer, to … the first purified product, it took 19 days
Marc-André D’Aoust, executive vice president at Medicago
“From the moment we had the sequence on our computer, to the moment we [had the] first purified product, it took 19 days,” says Marc-André D’Aoust, executive vice president of the company. This speed, which compares with five to six months for a conventional egg-grown vaccine, is something that will be hugely advantageous in potential future pandemics.
Nicotiana benthamiana — the workhorse of molecular farming
Researchers and companies working with whole plants have largely settled on Nicotiana benthamiana (N. benthamiana); a plant that is indigenous to Australia and a close relative of the tobacco plant.
“Nothing’s quite as good [as N. benthamiana],” says Lomonossoff. “[Its] great attraction is [that] it has a defective plant silencing system … it doesn’t try and counteract expression levels, which a lot of plants will naturally tend to do.” This means, when infected by bacteria, N. benthamiana has no ability to fight back and stop the bacteria hijacking its protein production machinery. As a result, yields of the protein of interest will be particularly high.
N. benthamiana is also easy and quick to grow.
“Plants grow to about 10–12 feet and their leaves are enormous,” says Julian Ma, director of the Institute for Infection and Immunity at St George’s University of London. It is also not a food crop, which is important, particularly in Europe, where there are concerns about genetically-modified organisms reaching the food chain.
After starting out with the alfalfa plant, owing to the ease at which it can be propagated, in 2005, Medicago also moved to using N. benthamiana plants. Now, in addition to its COVID-19 vaccine, it has completed phase II clinical trials for a pandemic flu vaccine and phase I trials for both rotavirus and norovirus vaccines.
The vaccines consist of plant-produced, virus-like-particles (VLP), which, explains Lomonossoff, have a structure similar to the original virus but are not infectious because they do not contain the genome. Lomonossoff has also developed several plant-produced VLP vaccine candidates, including one for polio.
Biopharmaceuticals from moss
However, not all molecular farming is based on methods that require growing whole plants, or indeed uses typical flowering plants.
Ralf Reski, from the University of Freiburg in south west Germany, is the co-inventor of the moss bioreactor that produces biopharmaceuticals using a specific moss called Physcomitrella patens and, Reski says, combines “the best of two worlds”.
“We have a plant, and we have containment and highly controlled conditions.”
The moss grows in an aqueous salt solution and expresses the protein being produced as a pharmaceutical directly into the growing medium, so it can be harvested without destroying the moss cells, making for a simpler purification process. Unlike mammalian CHO cell cultures, the moss does not require growth regulators or antibiotics, just light.
We find a very high batch-to-batch stability … with batches more similar than those for medicines produced in non-plant systems
Ralf Reski, co-inventor of the moss bioreactor, University of Freiburg, Germany
“We also find a very high batch-to-batch stability … [with batches] more similar than [those for] medicines on the market produced in non-plant systems,” says Reski.
His biotech company, Eleva, has completed a phase I clinical trial for its first drug candidate moss-aGal (agalsidase) to treat deficiency of the enzyme a-galactosidase, which causes Fabry disease, a rare genetic lysosomal storage disorder. They found their moss-produced enzyme had superior pharmacokinetics to the same enzyme made in mammalian cells.
However, one issue all plant-based methods face lies in the differences in glycosylation patterns in plant and animal cells.
Many biological drugs are glycoproteins, which means the proteins are covered with oligosaccharide chains (glycans), a process that occurs post-translation. This surface sugar coating is often important in forming the correct 3D protein conformation and, in membrane proteins, it plays a role in controlling how cells interact with other cells and molecules. In short, any differences in glycosylation patterns can affect the efficacy of a biological drug.
“[There are] specific sugars that you only find in plants, and others you only find in animals,” explains Reski. To counter this, Reski has genetically engineered the moss to ‘humanise’ the glycosylation process. He has deleted two genes that code for enzymes that add plant-specific sugars and added six genes that introduce human or mammalian sugars.
Those working with N. benthamiana have also engineered the plants to better match human glycosylation patterns, as well as to improve the product yield. Lomonossoff’s lab created a technology called Hypertrans, which is now used by Medicago and allows them to produce between 5mg to 5g of product per kilogram of plant material. The Hypertrans system delivers not only the genetic code for the protein but additional genetic instructions that speed up the translation of the RNA produced from the gene.
Besides the advantages of price and efficiency, Reski has also found that, sometimes, the plant-produced product is superior to production in CHO or other cells.
“We think [they are] sometimes biobetter, not biosimilar,” he suggests. “We found, for example, in antibodies which kill tumour cell lines, that there was a very specific sugar residue missing in our antibodies made in moss, and this helped — it was 40 times more efficient in killing these tumour cell lines[1].”
Biologics in the developing world
One area where plant-based pharmaceuticals could be particularly important is in the developing world, where access to expensive vaccines or antibody drugs is limited.
To grow plants you need sunlight, water and soil, you don’t need some complicated medium
Julian Ma, director of the Institute for Infection and Immunity, St George’s University of London
“You can get into the manufacturing game with a much lower investment, so developing countries are now able to start thinking about establishing biologics manufacturing facilities,” says Ma. “To grow plants, you need sunlight, water and soil, you don’t need some complicated medium.”
Several countries are already starting plant-based pharmaceutical production. A start-up based in Thailand, Baiya Phytopharm, has developed a COVID-19 vaccine using N. benthamiana. The company started phase I trials in September 2021 and hopes to gain regulatory approval by the third quarter of 2022.
In Brazil, Canadian biopharmaceutical company PlantForm Corporation and leading Brazilian biotechnology company Axis Biotec are collaborating to develop a plant-based biosimilar for pembrolizumab (Keytruda; Merck), the antibody immunotherapy drug used to treat many types of cancer.
In his lab, Ma has also been working on an antibody to neutralise HIV, expressed in a glycoengineered line of N. benthamiana plants.
“I don’t think in South Africa [where HIV prevalence is around 20%], you could afford anything near the price that antibodies are at moment, so that’s one of the things that we’ve been working on, and I think it’s really quite exciting,” he says[2].
Researchers have also been experimenting with the use of edible crops, such as grains, he adds.
“It turns out that maize is a brilliant crop because you can harvest the maize seeds, and those maize seeds are natural protein storage organs, and you can store your biologic in a sort of semi-processed form for years at ambient temperatures when you keep them dry,” explains Ma.
In 2021, a team from the University of Tokyo completed a phase I trial of its oral rice-produced cholera vaccine (MucoRIce-CTB), which is administered by powdering the rice and drinking it suspended in a phosphate-buffered saline solution[3]. Results showed increased antibody concentrations without serious adverse events.
One issue for Europe will be legal restrictions against growing GMOs. Gleba says Nomad Bioscience is currently growing plants in pre-existing greenhouse facilities, originally created for more conventional crops, in Spain: “the only European Union country that is not averse to commercial scale growing of GM plants”.
Ma fears that even though the crops are not intended for the food chain, the issues around GMOs in Europe may slow down uptake of the technology. Post-Brexit, the UK will now allow field-trials of gene-edited crops, provided the plants are contained in greenhouses or other enclosed spaces.
So far, big pharma has been relatively slow to show interest in plant-based technology owing to being heavily invested in current production methods.
“It’s like turning a tanker, to get them to go to a different manufacturing platform,’ says Ma.
But, Gleba is convinced that, in the long term, plants will play an increasingly important role in how biopharmaceuticals are made.
“It’s a very democratic direction for technology,” he says.
The ability to produce drugs with little infrastructure has also caught the attention of the National Aeronautics and Space Administration, which is looking for technologies that might assist missions to Mars. For example, Gleba explains, one little leaf of the N. benthamiana plant can provide six months of therapy for six patients with hepatitis C.
So, if the technology does not continue to take off on earth, perhaps, one day, plants will become the go-to pharmaceuticals in space.
- 1Schuster M, Jost W, Mudde GC, et al. In vivo glyco-engineered antibody with improved lytic potential produced by an innovative non-mammalian expression system. Biotechnol. J. 2007;2:700–8. doi:10.1002/biot.200600255
- 2Moore CM, Grandits M, Grünwald-Gruber C, et al. Characterisation of a highly potent and near pan-neutralising anti-HIV monoclonal antibody expressed in tobacco plants. Retrovirology. 2021;18. doi:10.1186/s12977-021-00560-6
- 3Yuki Y, Nojima M, Hosono O, et al. Oral MucoRice-CTB vaccine for safety and microbiota-dependent immunogenicity in humans: a phase 1 randomised trial. The Lancet Microbe. 2021;2:e429–40. doi:10.1016/s2666-5247(20)30196-8


