
Mclean/Shutterstock.com
It could be argued that the adverse impact of medicines manufacture on the environment is an unfortunate, but necessary, side effect of the drive to produce life-saving medicines. But this reasoning is seriously flawed. The pharmaceutical industry produces significant amounts of waste products and pollutants — more than any other chemical industry — that are harming not only the environment, but our health too.
However, there is hope. Over the past few decades, the field of ‘green chemistry’ — the design of chemical products and processes that reduce or eliminate the generation of hazardous substances — has been moving fast, and pharmaceuticals are a vital part of this.
When a medicine is developed, the two highest priorities are that it works effectively and that it is safe. However, there is an increasing drive towards developing pharmaceuticals that achieve this, while also leaving a smaller environmental footprint from their manufacture.
“Across the board in the chemistry field, we’re now thinking about raw materials, processes, product and end of life,” says James Clark, director of the Green Chemistry Centre of Excellence at the University of York. “With all these factors playing their part in how we consider sustainability, or whether a product or process is ‘green’.”
Green chemistry principles are comparatively simple to apply and often result in a greener process, but on their own they don’t guarantee it
Claire Adjiman, a chemical engineer at University College London
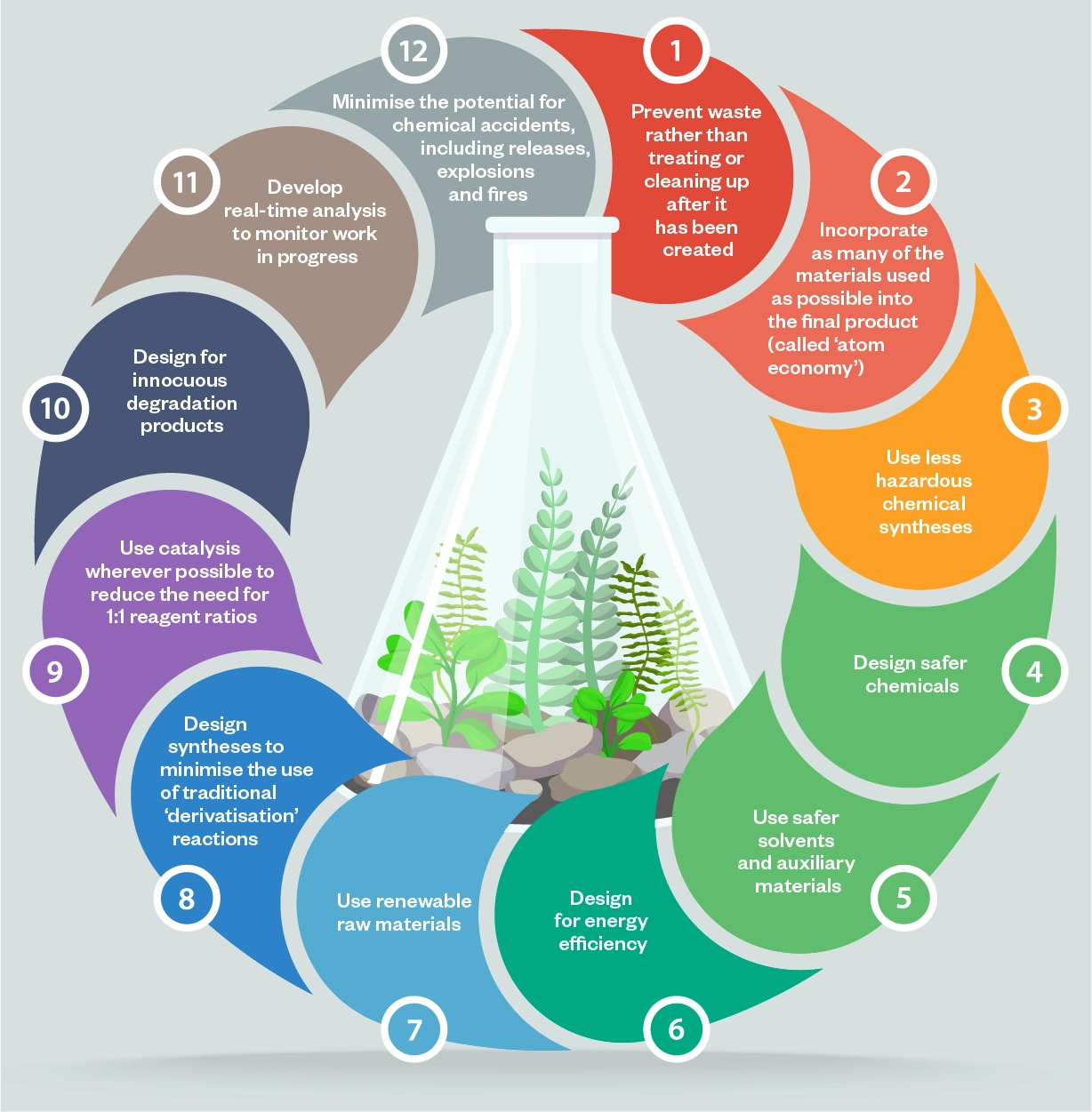
Unsurprisingly, it is all highly complex. The ‘12 principles of green chemistry’, drawn up by the American Chemical Society (ACS; see Figure), are broadly applicable. However, they also need to be put into context; it is no good achieving a very high ‘score’ in one area if, ultimately, it makes the product more damaging overall. Claire Adjiman, a chemical engineer at University College London, explains: “You have to take a holistic view of the manufacturing processes, factoring in overall waste generation, carbon emissions, other pollutants and so on. For instance, it may be better to select a reaction which produces more impurities, but impurities that are easier to remove.” This balancing act is a huge challenge within the manufacturing industry, she adds. “Green chemistry principles are comparatively simple to apply and often result in a greener process, but on their own they don’t guarantee it.”
Pharmaceuticals are particularly difficult cases, says Adjiman. “The molecules themselves are more complicated and putting them together takes many steps. You have to ensure a very high level of purity (a higher level than, for instance, you’d require for plastics). Then you need the right molecules in the right form for optimum absorption; the best way to take a medicine is a tablet, and that means it’s complex to manufacture. All this makes it much harder to follow through with a holistic green process.”
Industry take-up
Like corporate social responsibility, green chemistry is a field in which it is easy for big firms to make big declarations (and to employ a range of different metrics and measurements) without taking much action. Companies also tend to refer enquiries to the corporate website rather than answer questions directly. But changes are happening, some of which have become part of the mainstream.
Pharmaceutical companies have switched the manufacturing processes of several well-established medicines — including the diabetes drug sitagliptin, the malaria treatment artemisinin and the painkiller ibuprofen — to less environmentally damaging ones in recent years.
At the University of Nottingham, the GSK Carbon Neutral Laboratories for Sustainable Chemistry project officially opened its laboratory in February 2017, backed by a £12m fund from the pharmaceutical company. The building is estimated to use around a third of the power of a conventional chemistry laboratory and consume far less water.
The ACS Green Chemistry Institute Pharmaceutical Roundtable, which was set up in 2005, now has more than 20 members, including AstraZeneca, Pfizer, Johnson & Johnson, Merck and Eli Lilly. Its activities include maintaining a research programme and the production of guidelines and standards across a range of chemical processes, as well as hosting the annual Green Chemistry Challenge Awards, intended for ‘groundbreaking scientific solutions to real-world environmental problems’. The 2021 winners included Merck, for developing a more sustainable manufacturing process for the chronic cough medicine gefapixant citrate (see Box) and Bristol Myers Squibb, for a new class of sustainable reagents (substances used to cause a chemical reaction).
Box: Gefapixant citrate — developing a greener synthetic pathway
Merck was awarded the Green Chemistry Challenge 2021 award for greener synthetic pathways for developing a green and sustainable commercial manufacturing process for gefapixant citrate — an investigational medicine for chronic cough.
The initial manufacturing process involved many steps and had a high process mass intensity (PMI) — a measure of process efficiency for biopharmaceutical production — of 366.
The new process has a PMI of 88, a greater than four-fold improvement. The main innovations are:
- Implementation of a highly efficient two-step methoxyphenol synthesis;
- An innovative diaminopyrimidine synthesis, using a hybrid flow-batch process;
- A simplified direct sulfonamide synthesis;
- A novel and robust salt metathesis approach to consistently deliver the correct salt form with high productivity.
The new process also led to a significantly higher product yield and a six-fold reduction in raw material costs. In addition, the alkylation step, which involved highly hazardous chemicals, was replaced and energy-saving processes that reduced carbon dioxide and carbon monoxide emissions were implemented.
Merck was a winner in 2020 too; this time for a more sustainable, cost-effective and safer manufacturing process for the antiviral drug uprifosbuvir. Merck developed a catalyst that can be used to synthesise uprifosbuvir with very high purity in just two reaction steps and says the new process results in greater than 75% improvements in process mass intensity — a measure of process efficiency for biopharmaceutical production — energy use, water depletion and other metrics, compared with the first-generation synthesis pathway.
The manufacturers of generic drugs are starting to work in [the green chemistry] space too
James Clark, director of the Green Chemistry Centre of Excellence at the University of York
“The manufacturers of generic drugs are starting to work in [the green chemistry] space too,” Clark adds. “That’ll be interesting in the future. There’s an assumption that these firms are ‘cheap and cheerful’ but I think they are showing signs of being rather more responsible.”
Three areas of focus are solvents, biocatalysis and continuous manufacturing.
Solvents
“Solvents are a big hitter,” says Chris Price, a chemical and process engineer at the University of Strathclyde, Glasgow, who spent 14 years working in innovation and process development at GSK. Solvents are an essential part of the manufacturing process: they are the substances (which might be water) that can dissolve the different materials that are being used, and at the right temperature. “Solvents are one of the key tools the industry works with to ensure high quality: by changing the solvent [used]; for instance, you can ensure that a tablet holds together properly,” Adjiman explains.
Solvents are a target for green chemistry for several reasons. On average, making one kilogram of active pharmaceutical ingredient takes four kilograms of organic solvents (such as acetone), excluding water. Heating and cooling solvents also uses a huge amount of energy. “If you look at the amount of waste involved in manufacturing a product, in the oil industry it’s around 10%: in the pharmaceutical industry, there’s a 1000-fold difference, and most of that waste is solvent,” Price says. And most solvents are not recycled — they are sent off to be incinerated, which uses yet more energy and leaves more waste products. Water is not necessarily the right solution either, Adjiman points out. “You might think a process that uses water is greener than using a solvent that is more toxic, but you may end up with more waste, and more dangerous waste as a result.”
However, Price explains, over the past two decades, there has been a big industry shift to using less toxic or environmentally damaging solvents. “Around the turn of the millennium, GSK and other firms started to put their heads above the parapet to create ‘red/amber/green’ lists of solvents, encouraging staff developing drugs to move to the green lists. We’re now starting to see much less use of those undesirable solvents and you see copies of the list pinned up by people’s desks. It’s had a huge impact because people agreed a consensus and stuck to it.”
Biocatalysis
Catalysts are the chemicals that can make a chemical reaction happen or speed up one that is already happening. Biocatalysis builds on the fact that a huge range of ‘natural’ catalysts already exist, in the form of enzymes — the proteins that make all kinds of biological processes happen. Over the past few decades, chemists have been using enzymes in several different industrial and commercial areas, and biocatalysis has become firmly established as part of the drive towards greener pharmaceuticals as well.
Biocatalysts are either existing enzymes or, often, new enzymes, which have been manufactured by introducing mutations in the amino-acid chains that make up the proteins. “It’s a numbers game,” explains Ahir Pushpanath, team leader at speciality chemicals and sustainable technologies company Johnson Matthey. “There are millions of natural enzymes. You need to be able to collect a range … that are potentially capable of catalysing this particular reaction.” Enzymes are considered preferable not just because they are made of renewable, biodegradable materials, but also because they do not use as much energy as synthetically derived catalysts. The research process is largely computer-based as well, which saves laboratory time, work and reagents (although it also involves sifting through huge numbers of potential candidates).
Pushpanath points to several examples, including the diabetes drug sitagliptin and heart failure treatment sacubitril, whose greener manufacturing has been made possible through biocatalysis. “That’s because of a particular class of enzyme: transaminases, but there are countless others, and obviously we’re working on more.”
Continuous manufacturing
Several companies are also moving to ‘continuous manufacturing’, with Eli Lilly one of the leaders in the field. This is the way that most chemical industries work, with all parts of the product being manufactured at the same time as opposed to ‘batch manufacturing’, where each stage of production is performed separately and tested at the end.
The enemy of sustainability is waste, and this process is much less wasteful
Chris Price, a chemical and process engineer at the University of Strathclyde, Glasgow
As a process, it is not necessarily more sustainable or greener but, in practice, Price explains, it tends to work out that way; partly through the chemicals that are selected but also because it should produce less waste. “The enemy of sustainability is waste, and this process is much less wasteful. It should give a consistently higher standard of product, whereas pharmaceutical products that don’t meet the specification are incinerated. It’s resource-efficient: everything that’s bought is used in the product. And another big area of waste in the sector is time-expired materials, so it’s very helpful if you can develop a supply chain that delivers consistently, with very few materials going out of date at any stage in the process.”
Moving into a new century
“Where the pharmaceutical industry led the way is in terms of green chemistry metrics: measuring and ways to measure, for instance, the different components, such as solvents. That’s been very useful in industry more widely,” says Clark. He also points out that a lot of the work that is going on in industry is conducted behind closed doors and is never revealed to the public. “The strength of the effort going in does seem to vary, but they’ve come up with some fantastic innovations; the problem is that they’re not often publicised, which is very frustrating. In terms of the process chemistry, they’ve invented routes we don’t see. The ones that are publicised aren’t nearly as many as the ones that have been produced.”
But there’s also a huge scope for moving forward. Both Clark and Price argue that, in many ways, the pharmaceutical industry hasn’t moved on for more than a century. “I’ve been to manufacturing facilities where the processes have some very nice equipment and everything looks very clean and efficient, but if you look at the actual chemical reactions going on, you’re seeing some very old chemistry, which tends to be very inefficient,” Clark says. Price takes this further: “Pharmaceutical manufacturing is the last bastion of 19th century technologies. The equipment is great but my grandfather would have recognised what is going on there. There’s the opportunity to make real improvements.”


