Mclean/Shutterstock.com
When patients began reporting lingering symptoms following COVID-19 infection, just a few months into the 2020 pandemic, initially they were ignored.
“We owe a debt to patients here,” stresses Amitava Banerjee, professor of clinical data science at University College London.
“Remember in May 2020 — it was patients who defined this, not doctors, not researchers, not politicians. Their mobilisation is what has forced the World Health Organization to have a consensus definition, forced research funding calls, and people like me on the clinical research side to take a look.”
It was patients, too, who founded the name ‘long COVID’, a term that has now been adopted by major UK healthcare stakeholders, including the National Institute for Health and Excellence (NICE) and the National Institute for Health Research (NIHR).
NICE has since defined long COVID as ongoing COVID-19 infection where symptoms continue between 4–12 weeks after infection, and post-COVID-19 syndrome where symptoms persist beyond 12 weeks.
“But not only is [long COVID] a totally new disease in terms of definition, it’s a totally new disease in terms of all the tools we would use for diagnosis, treatment or rehabilitation of an established condition,” Banerjee adds.
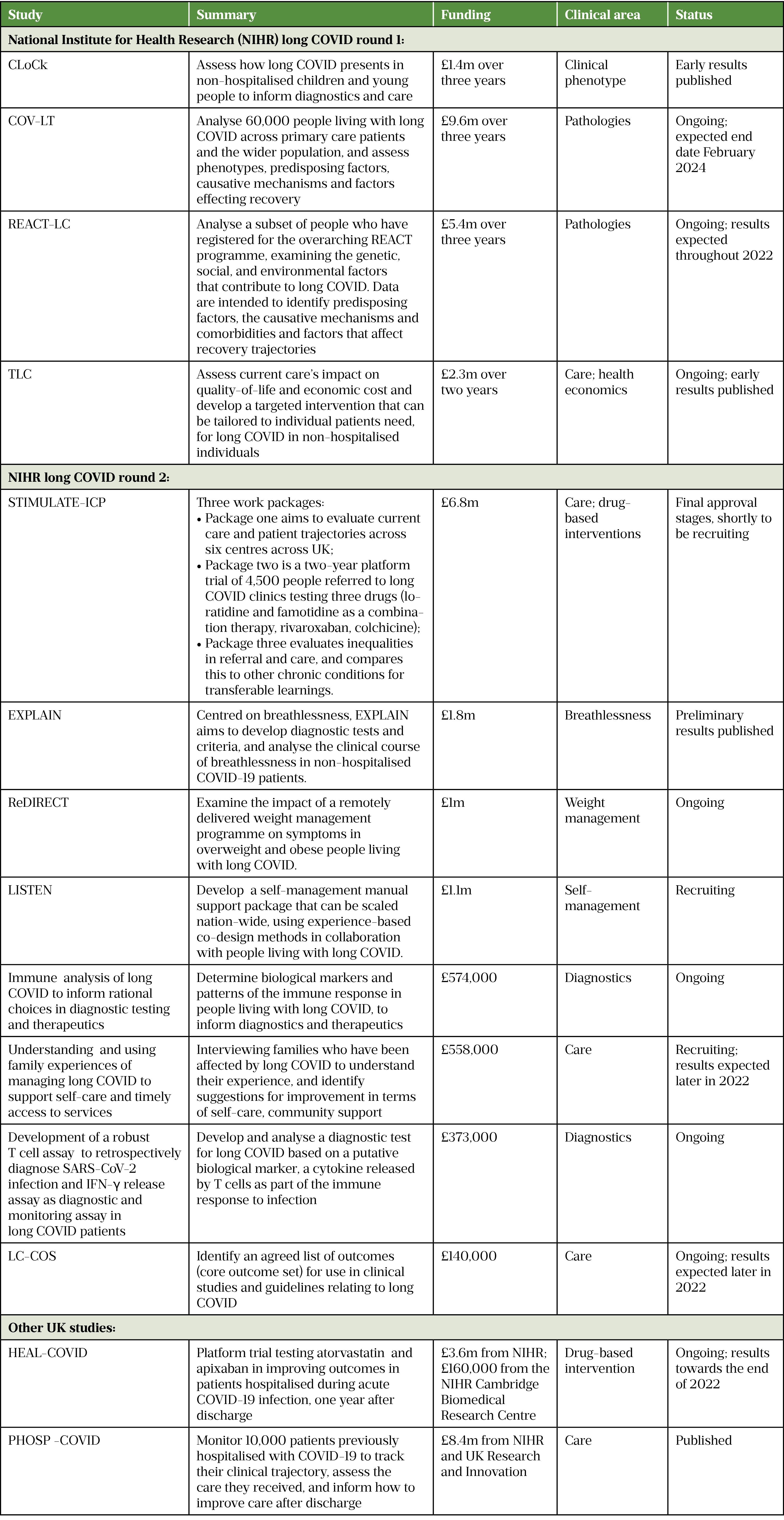
We are still developing that toolkit. The NIHR has offered £18.5m to fund four large ongoing studies aiming to lift the lid on the condition, while a second £19.6m fund is supporting 15 more studies, which are mainly aimed at improving diagnostics and treatment (see Table).
It was patients who defined long COVID, not doctors, not researchers, not politicians
Amitava Banerjee, professor of clinical data science at University College London
Even though it has not invested the most in long COVID research — the United States has allocated US$1.15bn (around £850m) over four years — the UK is leading the way on the global research stage owing to public willingness to respond to surveys and studies, plus the ability to connect this back to national patient data.
Advancing diagnostics
The issue with long COVID is that there is no definitive test for it. Diagnosis is based on symptoms and — although patient survey data indicate fatigue, breathing issues and cognitive dysfunction as the more prevalent and debilitating symptoms — there are 203 total symptoms associated with long COVID[1,2]. This suggests that long COVID might actually be a set of diseases, rather than one distinct entity.
Studies are assessing diagnostic tools, such as novel MRI techniques, to identify the reason for breathlessness or detect lasting lung damage, which current tests fail to pick up[3–5]. Other studies are looking to develop laboratory-based assays, analysing immunological markers in blood samples for reliable biomarkers[6].
Diagnostic criteria will also be honed as symptom profiles start to identify patterns or groupings. UCL Great Ormond Street Hospital (GOSH)’s ‘Children and young people with long COVID’ — or ‘CLoCK’ — study, part of the first round of NIHR funding, has begun to do that and, at 18,000 strong, is one of the world’s biggest datasets on long COVID for children and young people.
By comparing ongoing symptoms at three months after COVID-19 infection in nearly 7,000 children and young people with those of a control group, the study’s early findings have highlighted useful possible parameters for diagnosis, such as the number of symptoms present[7].
More than half of the control group who received a negative PCR test reported at least one symptom associated with long COVID.
“But, if you look at [children who reported] three symptoms, it’s about 30% of test positives and 15% of test negatives. If you look at 5+ symptoms, I think it was something like 13% of test positives and 6% of the test negatives,” recounts Terence Stephenson, Nuffield professor of Child Health at UCL GOSH and a co-investigator in the study.
“So you start to get a bigger gap when you ask about multiple symptoms — that does seem to be a discriminator.”
Advancing mechanistic understanding
One of the biggest hurdles in long COVID research is the black box behind the symptoms — the underlying causative mechanisms.
“We can recognise the clinical syndrome,” acknowledges Helen Ward, professor of public health at Imperial College London. “But we also know it will involve multiple pathologies.
“The evidence, such as it is at the moment, is that it may be persistent virus, when they’re not clearing the virus, not uncommon in viral illnesses. Then there’s just the inflammatory response itself, which becomes more like an immune or autoimmune disorder primed by the infection, leading to ongoing inflammation in multiple organ systems. There is also evidence of people with clotting disorders that are developing as a result of infection, and then you get possibly microclots.”
We recognise the clinical syndrome but we also know it will involve multiple pathologies
Helen Ward, professor of public health at Imperial College London
The study is looking to open up that black box, with its database of people who have tested positive and negative to Sars-CoV-2 infection, both hospitalised and non-hospitalised, severely and mildly unwell, and even asymptomatic cases[8].
Ward is part of Imperial College London’s ‘Real time assessment of community transmission’ — or ‘REACT’ — study programme, which has been monitoring more than 3 million people since May 2020.
As part of REACT’s long COVID (REACT-LC) arm, the group has identified 10,000 people who have tested positive for Sars-CoV-2, both with and without long COVID, to examine more closely.
“What we’re now doing is going back to people, calling them up to clinic as part of the LC study,” Ward explains.
“We’ve been doing detailed clinical, physical assessments and taking blood, which has been going for whole genome sequencing and other genetic and immunological analyses.”
This has led the researchers towards several pathways involved in the immune response.
“For instance,” Ward explains, “there are suggestions of alterations in the interleukin pathway. If you can identify particular genetic changes in that pathway that are associated with long COVID, then we may be able to identify the precise disorder and suggest trial of existing drugs, such as interleukin inhibitors.”
Anti-inflammatories, such as dexamethasone, have been crucial in the treatment of hospitalised patients with COVID-19, so it is not surprising that inflammation plays a role in long COVID. A second pathway highlighted by emerging research is coagulopathy, with recent findings of increased levels of microclots in long COVID patients’ plasma[9].
The South African team responsible for the findings suggests microclots contribute to long COVID symptoms such as breathlessness, sleep disturbances and muscle weakness, acting to block microcapillaries and interfere with oxygen exchange. Molecular analysis of microclots identifies them as clumps of clotting factors that contain trapped inflammatory molecules and, crucially, an anti-clotting inhibitor, alpha(2)-antiplasmin.
When the clotting response is triggered, it is regulated by the plasmin-antiplasmin system, with plasmins acting to dissolve blood clots and anti-plasmins blocking their action; together, the two form a push–pull relationship to maintain a healthy balance. The research team suggests microclotting events could be caused by an imbalance in the plasmin-antiplasmin pathway, with the plasmin inhibitor, alpha(2)-antiplasmin, inside the microclots making them especially resistant to dissolution.
Advancing treatment
Mechanistic research, however, remains preliminary, with no consensus hypothesis yet explaining long COVID pathology or allowing clinical sub-classifications.
Current lack of diagnostic and mechanistic specificity is causing confusion in areas of clinical overlap. In particular, the blurred line between long COVID fatigue and post-viral fatigue syndromes, such as myalgic encephalomyelitis (ME) or chronic fatigue syndrome (CFS), is a hot topic in the patient and clinical community (see Box).
Nevertheless, emerging disease or not, treatment is still tasked to healthcare professionals for the more than 1.5 million people who self-report experiencing long COVID symptoms in the UK.
Anyone with ongoing symptoms of long COVID can access clinical assessment and diagnosis at one of the UK’s 90 long COVID clinics, established with £100m of additional funding as part of ‘Long COVID: the NHS plan for 2021/2022’, published in June 2021[10].
However, waiting times are long and access varies across the country, with 64% of people waiting at least 15 weeks for an initial assessment in the south east of England compared with 12% of people in the north west[11]. Questions probing how standardised access to care is across the country and identifying gaps in support are the focus of ongoing NIHR-funded studies (see Table).
But in the same way as many chronic conditions, treatment plans tend to emphasise self-management and working out how to live with the symptoms — not fix them — and the absence of drug-based therapies is palpable.
“There is a very desperate clinical and patient community who, for coming up to now two years, have been unable to do anything,” Banerjee says. “There’s huge potential for bad practice and bad science, where people are saying you should have this drug, take this supplement, have this treatment, or do this test, because in the absence of evidence, people have nothing else[12]. So it’s important we get good evidence out there.”
Despite these knowledge and treatment gaps, studies addressing drug-based interventions for long COVID are few and far between.
There is a very desperate clinical and patient community who, for coming up to now two years, have been unable to do anything
Amitava Banerjee, professor of clinical data science at University College London
Banerjee is co-lead investigator of STIMULATE ICP (‘Symptoms, Trajectory, Inequalities and Management: Understanding Long-COVID to Address and Transform Existing Integrated Care Pathways’), an overarching package of NIHR-funded studies that includes a platform trial arm, testing repurposed drugs in non-hospitalised patients who are referred to a long COVID clinic[13].
The platform trial is designed to adaptively test more drugs as the evidence base builds when findings from studies such as REACT-LC become available, and has structures in place to translate positive results to clinic in rapid time.
Currently at the final stages of approval, STIMULATE ICP is planning to recruit 4,500 people and plans to investigate three interventions for the treatment of long COVID: antihistamines loratidine and famotidine as a combination therapy (building on emerging evidence that antihistamines have a role to play in taming the inflammatory response associated with COVID-19 infection and development of long COVID); anticoagulant rivaroxaban (given the emerging findings around micro-clots); and the anti-inflammatory colchicine (to target lung and heart inflammation)[14,15].
Another platform trial fringing long COVID research, set up from an earlier round of NIHR funding, is ‘Helping alleviate the longer-term consequences of COVID-19’ (HEAL-COVID), which trials drugs in previously hospitalised COVID-19 patients[16].
“Most people, when they leave hospital having recovered from acute COVID, think ‘this is great, I’ve made it out of hospital, I’m alive, I’m going to be fine’,” says Charlotte Summers, professor of intensive care medicine at the University of Cambridge and chief investigator of HEAL-COVID.
However, the statistics show this is not the case, with 29% of hospitalised COVID patients readmitted to hospital within six months of discharge and a 12% mortality rate.
“HEAL-COVID was [designed] to see if we could reduce the number of people who die and end up back in hospital over that first year after discharge from hospital with acute COVID infection — we are trying to prevent people from getting long COVID, effectively.”
HEAL-COVID randomises patients to receive either the anticoagulant apixaban for two weeks from the day of hospital discharge, owing to evidence suggesting this period of time is linked to the peak incidence of post-COVID clotting events, or atorvastatin, given for its effects on vascular inflammation, for 12 months. With a primary endpoint of hospital-free survival, the study can be conducted remotely to maximise participation and nearly 1,000 subjects are currently active. Results are anticipated towards the end of 2022 (see Table).
By now, more than two years since the pandemic first swept the UK, we are acclimatised to the stress that each COVID-19 wave adds to us and the healthcare service. But the rising tide of long COVID is a different threat requiring different sandbags. Results from long COVID research cannot come soon enough.
Box: Myalgic encephalomyelitis and chronic fatigue syndrome — what does long COVID mean for us?
“When people hear about long COVID in the news as if it’s a new condition and no one’s come across anything like it before, people who have been living with myalgic encephalomyelitis (ME) for many years find that difficult to hear,” says Peter Gladwell, clinical specialist physiotherapist and service lead at the Bristol ME Service, and a member of the British Association of Clinicians in chronic fatigue syndrome (CFS)/ME (BACME).
There are no drugs approved in the UK for fatigue in ME/CFS. The few medicines that are given to manage symptoms are rarely evidence-based in the ME/CFS population but translated under expert guidance from other conditions, such as neuropathic pain or nausea.
There are several reasons behind this — a lack of funding has left ‘fatigue science’ decades behind medical understanding of its pathway peers, such as pain or inflammation. This prevents drug–target identification and design of novel therapies.
In addition, the ME/CFS community comes with added layers of complexity that challenge drug–based therapy advancement.
“With ME/CFS it is difficult to gather a cohort with clear, documented evidence of an infective onset — people will often spot a couple of things, a complex pattern that triggers ME/CFS in their life, and it’s very difficult then to try and tease out to what extent these different triggers are perhaps creating a response within the host,” Gladwell explains.
“Whereas with long COVID, it’s a bit more obvious it’s a COVID-19 infection that is the significant factor and that documentation is really helpful for research.”
The sheer numbers of people with long COVID mean a subset of similar medical history can be recruited for clinical trials.
“Another reason is people with ME/CFS are often sensitive to medicines and can’t start at the dose someone else might,” Gladwell adds. “So then you don’t have a standard intervention that you can compare, because each patient has got to titrate up gradually.”
Although there are early indications of medicines sensitivity in the long COVID cohort, their size means they have an advantage in overcoming this complexity.
There is hope that the spotlight on long COVID will advance the understanding of fatigue and generate new therapies that can also benefit the ME/CFS community.
“But I think that whatever findings come from long COVID research will need to be re-tested in the ME/CFS population.”
- 1Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK : 3 March 2022. Office for National Statistics. 2022.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/3march2022 (accessed 3 Mar 2022).
- 2Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi:10.1016/j.eclinm.2021.101019
- 3New research studies to help diagnose and treat Long COVID funded by NIHR. NIHR. 2021.https://www.ox.ac.uk/news/2021-07-19-new-research-studies-help-diagnose-and-treat-long-covid-funded-nihr (accessed 2 Mar 2022).
- 4Frequently Asked Questions. STIMULATE-ICP. https://www.stimulate-icp.org/faq (accessed 2 Mar 2022).
- 5Grist JT, Collier GJ, Walters H, et al. The Investigation of Pulmonary Abnormalities using Hyperpolarised Xenon Magnetic Resonance Imaging in Patients with Long-COVID. 2022. doi:10.1101/2022.02.01.22269999
- 6Biological ‘fingerprints’ of long COVID in blood could lead to diagnostic test, say Cambridge scientists. University of Cambridge. 2021.https://www.cam.ac.uk/research/news/biological-fingerprints-of-long-COVID-in-blood-could-lead-to-diagnostic-test-say-cambridge (accessed 2 Mar 2022).
- 7Stephenson T, Pinto Pereira SM, Shafran R, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. The Lancet Child & Adolescent Health. 2022. doi:10.1016/s2352-4642(22)00022-0
- 8Real-time Assessment of Community Transmission (REACT) Study. Imperial College London. https://www.imperial.ac.uk/medicine/research-and-impact/groups/react-study/ (accessed 2 Mar 2022).
- 9Pretorius E, Vlok M, Venter C, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20. doi:10.1186/s12933-021-01359-7
- 10Long COVID: the NHS plan for 2021/22. NHS. 2021.https://www.england.nhs.uk/coronavirus/publication/long-covid-the-nhs-plan-for-2021-22/ (accessed 2 Mar 2022).
- 11COVID-19 Post-Covid Assessment Service. NHS. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-post-covid-assessment-service/ (accessed 2 Mar 2022).
- 12van der Togt V, Mcfarland S, Esperti M, et al. Promotion of non-evidence-based therapeutics within patient-led Long COVID support groups. Nat Med. 2021;27:2068–9. doi:10.1038/s41591-021-01589-y
- 13STIMULATE-ICP: Understanding long COVID to improve diagnosis, treatment and care. NIHR. https://www.arc-nt.nihr.ac.uk/research/projects/stimulate-icp-improving-diagnosis-treatment-and-care-of-long-COVID/ (accessed 2 Mar 2022).
- 14Glynne P, Tahmasebi N, Gant V, et al. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2021;70:61–7. doi:10.1136/jim-2021-002051
- 15May BC, Gallivan KH. Levocetirizine and montelukast in the COVID-19 treatment paradigm. International Immunopharmacology. 2022;103:108412. doi:10.1016/j.intimp.2021.108412
- 16HElping Alleviate the Longer-term consequences of COVID-19 (HEAL-COVID): a national platform trial. HEAL-COVID. https://heal-covid.net/about/ (accessed 2 Mar 2022).
- 17Stephenson T, Shafran R, De Stavola B, et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study). BMJ Open. 2021;11:e052838. doi:10.1136/bmjopen-2021-052838
- 18Characterisation, determinants, mechanisms and consequences of the long-term effects of COVID-19: providing the evidence base for health care services. NIHR. https://fundingawards.nihr.ac.uk/award/COV-LT-0009 (accessed 2 Mar 2022).
- 19Therapies for Long COVID in non-hospitalised individuals: the TLC Study. University of Birmingham. https://www.birmingham.ac.uk/research/applied-health/research/long-covid/index.aspx (accessed 2 Mar 2022).
- 20Aiyegbusi OL, Hughes SE, Turner G, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114:428–42. doi:10.1177/01410768211032850
- 21Study launched to investigate diet treatment for long COVID. University of Glasgow. 2021.https://www.gla.ac.uk/research/coronavirus/headline_802258_en.html (accessed 2 Mar 2022).
- 22Listen Project on Long Covid. Chartered Society of Physiotherapy. 2021.https://www.csp.org.uk/documents/listen-project-long-covid (accessed 2 Mar 2022).
- 23Understanding experiences of Long Covid in families. University of Oxford. https://www.phc.ox.ac.uk/research/health-experiences/understanding-experiences-of-Long-covid-in-families (accessed 2 Mar 2022).
- 24Core Outcome Measures for Post-Covid condition/Long Covid. COMET Initiative. https://www.comet-initiative.org/Studies/Details/1847 (accessed 2 Mar 2022).
- 25PHOSP-COVID About. PHOSP-COVID. https://www.phosp.org/about (accessed 2 Mar 2022).
- 26Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. The Lancet Respiratory Medicine. 2021;9:1275–87. doi:10.1016/s2213-2600(21)00383-0