
National Reporting and Learning System (NRLS)
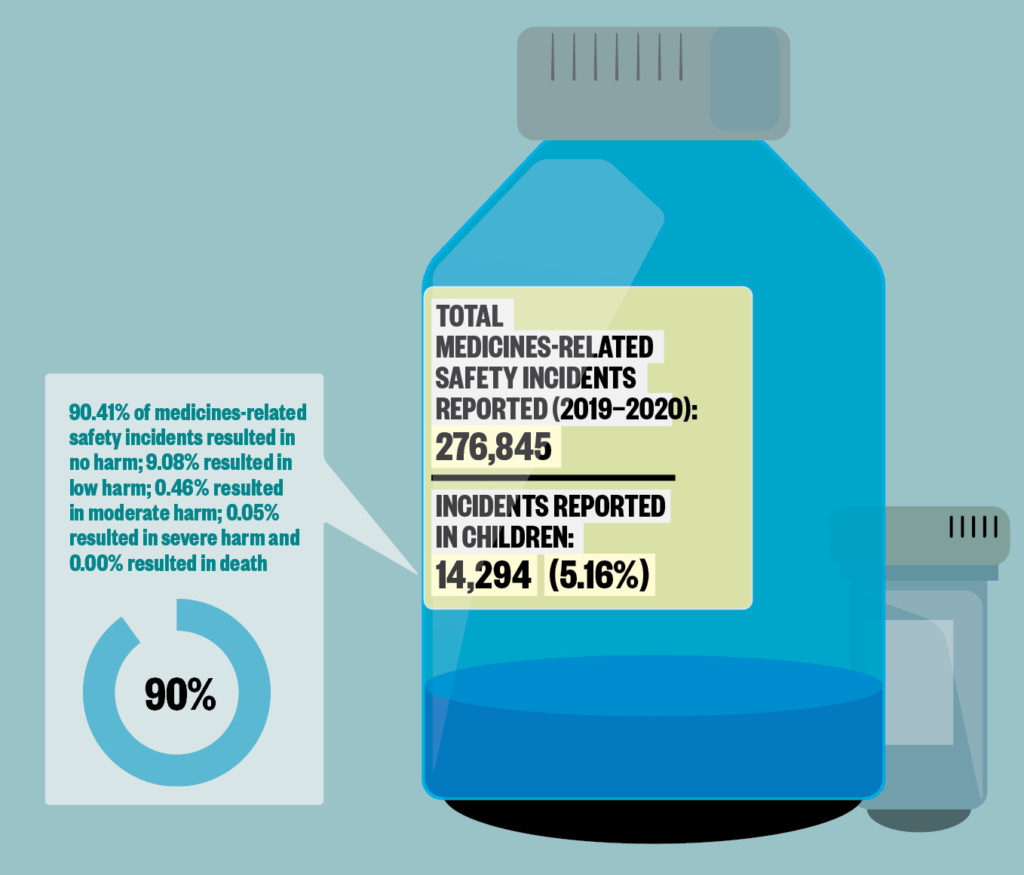
The NRLS is a voluntary reporting system for patient safety incidents, defined as any unintended or unexpected incidents that could have or did lead to harm. The number of incidents reported reflects reporting culture, and is not necessarily the actual number of incidents occurring.
Download the full PDF version here


Causes of incidents
Several factors that are specific to prescribing in children contribute to medicines safety incidents.
Individualised dosing: Doses or intervals not altered as children grow; small or large for age children not recognised; errors in calculations based on age, weight or body surface area; misplacement of decimal points; confusion around dosing equations; errors in weighing children.
Off-label or unlicensed prescribing: Lack of clear dosage information; multiple or unclear reference standards; trial and error dosage strategies.
Medicines formulations: Availability of appropriate presentations for children; incorrect dosage conversions: inappropriate prescribing in mL when multiple solution strengths are available; further manipulation to aid adherence.
Communication: Difficulties in accurate medicines reconciliation; inadequate communication of prescribing decisions and dose changes between care settings and to parents.
Experience of paediatrics: Lack of familiarity with doses and formulations for children and infants; not recognising differences in prescribing for children.
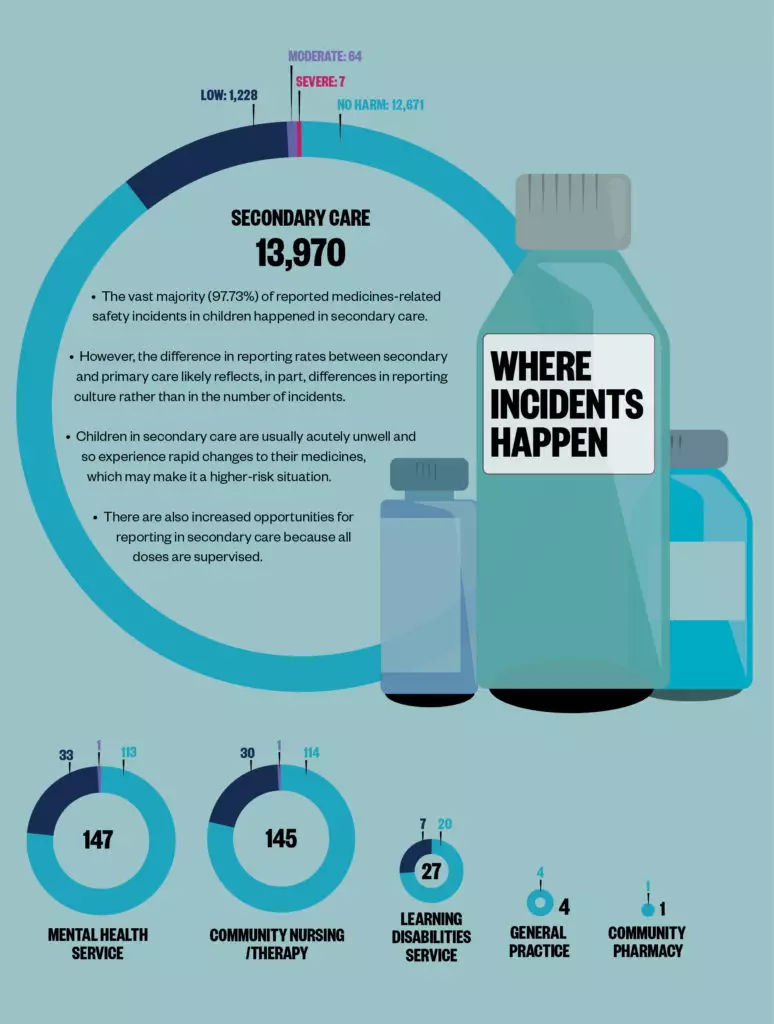
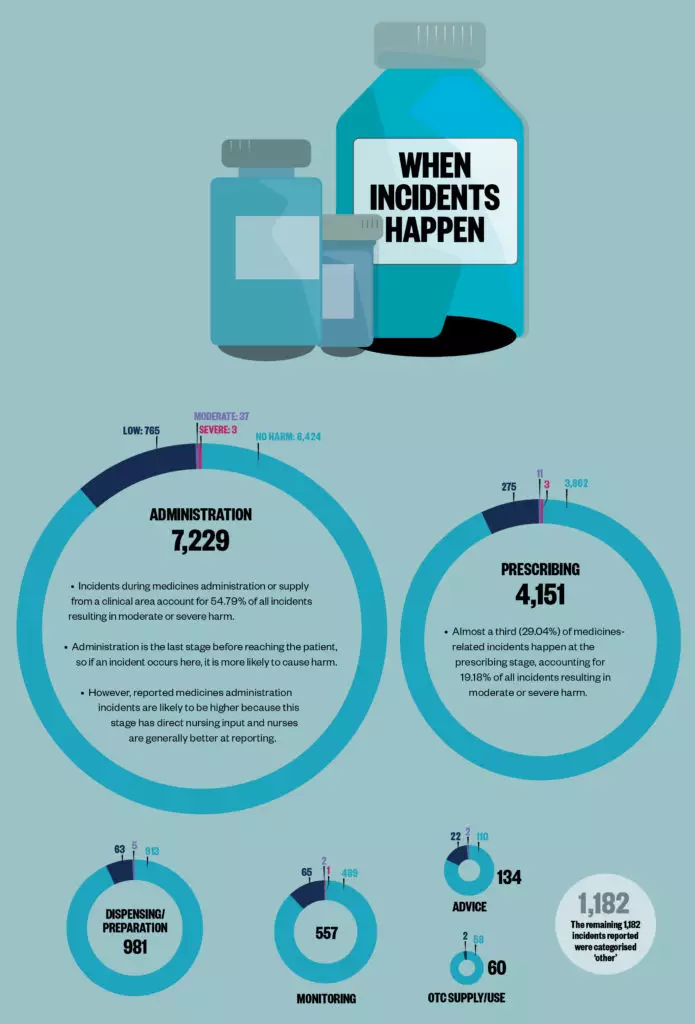
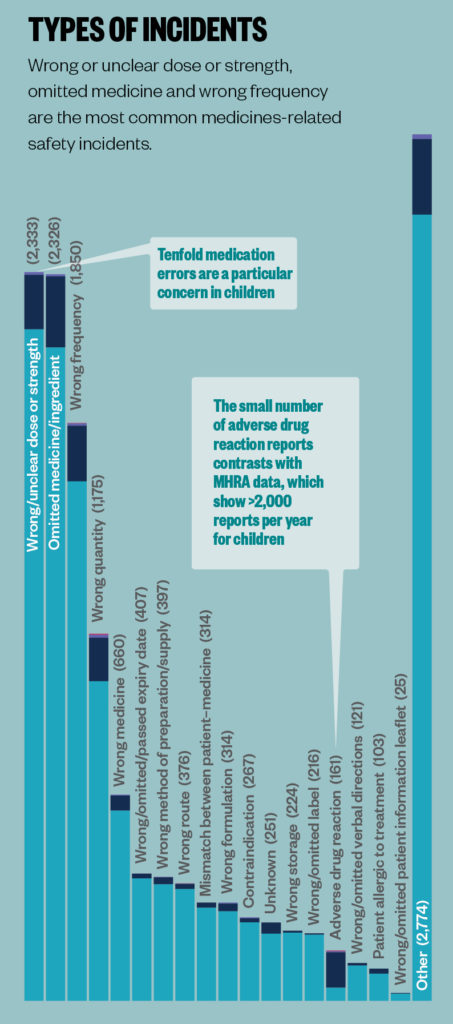
Sources: Data are for incidents in England reported to the National Reporting and Learning System between 1 April 2019 and 31 March 2020. Children are aged between 2 days and 17 years at the time of the incident. BMJ Open 2019;9:e028680; JAMA 2001;285:2114–20; Archives of Disease in Childhood 2020;105:e3.
Editorial advisers: Andy Fox, consultant pharmacist medicines safety and deputy chief pharmacist, University Hospital Southampton NHS Foundation Trust; Daniel B Hawcutt, senior lecturer in paediatric pharmacology at the University of Liverpool and honorary consultant in paediatrics at Alder Hey Children’s Hospital.
1 comment
You must be logged in to post a comment.


pharmacists, not paediatric trained, should be able to contact Paediatric pharmacists at their local Hospital