Shutterstock.com
By the end of this article, you should be able to:
- Name and summarise some of the more common cardiac arrhythmias that present in children;
- Appreciate and understand the pharmacotherapy for different arrhythmias;
- Recommend any necessary monitoring, including therapeutic drug monitoring.
Introduction
An arrhythmia is classified as an abnormal rhythm of the heart. In an arrhythmia, abnormal electrical signals are sent through the heart muscle (myocardium), which can lead to the heart beating too fast (tachycardia), too slow (bradycardia), or irregularly. This can lead to poor perfusion around the body, meaning that organs such as the brain and lungs may not function well and lead to damage1.
In terms of cardiac rhythm, the arrythmia can be described as regularly irregular — where the irregular rhythm of the heart is consistent; or could be classified as irregularly irregular where the rhythm has no distinct pattern.
These types of cardiac abnormalities are relatively common in children, with an estimated incidence of almost 1 in 4,000 births1. Most of these are supraventricular, with around half of children potentially experiencing their first episode as an infant; however, symptoms can be hard to detect, particularly in younger children. This can lead to a delay in diagnosis and contribute to ventricular dysfunction, heart failure and acute collapse2.
Paediatric arrhythmias can be congenital (present from birth) or acquired (developed over time). The underlying cause is commony idiopathic and common causes can also include:
- Congenital heart defects: structural abnormalities in the heart can disrupt its electrical pathways, leading to arrhythmias2;
- Infections: viral infections, such as myocarditis, can affect the heart’s electrical system;
- Genetic factors: some arrhythmias have a hereditary component, in an autosomal dominant manner, such as first-degree family members; therefore, a family history is ideal as this can increase a child’s risk;
- Metabolic disorders: conditions such as electrolyte imbalances, thyroid problems and certain medications can lead to arrhythmias3;
- Cardiac surgery: post-operative arrhythmias are very common, particularly junctional ectopic tachycardia (JET).
When managing cardiac arrythmias, pharmacists have an opportunity to input into therapeutic decisions and long-term management of the patient’s condition. Paediatric patients with cardiac arrythmias often have multiple comorbidities requiring safe and effective management of potential drug–drug and drug–disease interactions.
This article will provide an outline of the main types of arrhythmias, diagnosis and pharmaceutical treatment options. A working knowledge of ECGs will be helpful, but please read ‘Interpretation of electrocardiograms’ for more information on this.
Types of arrhythmias
Arrhythmias is a broad condition type with a multitude of subsets. They can be broadly categorised into bradyarrhythmia’s and tachyarrhythmia based on the heart rate. This can be further divided according to the origin, means of transmission and syndromes associated with it. The following represent a selection of the types of arrythmia most commonly seen in the paediatric population.
Supraventricular tachycardia
Supraventricular tachycardia (SVT) is one of the most common cardiac arryhthmias affecting the paediatric population4. It is characterised by rapid and regular heartbeats originating above the ventricles4. This often lasts for more than 30 seconds and can require termination if accompanied with haemodynamic instability5. SVT is an umbrella term for several conditions, including atrial fibrillation, atrial flutter, atrioventricular (AV) nodal re-entrant tachycardia and accessory pathway-mediated tachycardia. Exploring the pathophysiology and underlying mechanisms that contribute to the development of these conditions will give readers a greater understanding of the mechanism of treatment and treatment aims.
Electrophysical mechanisms
In the normal electrical conduction system, the sino-atrial node (SAN) starts the contraction sequence with the signal then travelling to the AV node, through the bundles of His (BH) and then through the purkinje fibers (the impulse also travels from right bundle branch to the anterior papillary muscle ensuring equal conduction time across the ventricles). In contrast, the pathophysiology of SVT involves abnormal electrical impulses arising within the atrium, AV node or accessory pathways, which bypass the normal electrical conduction system3,4.
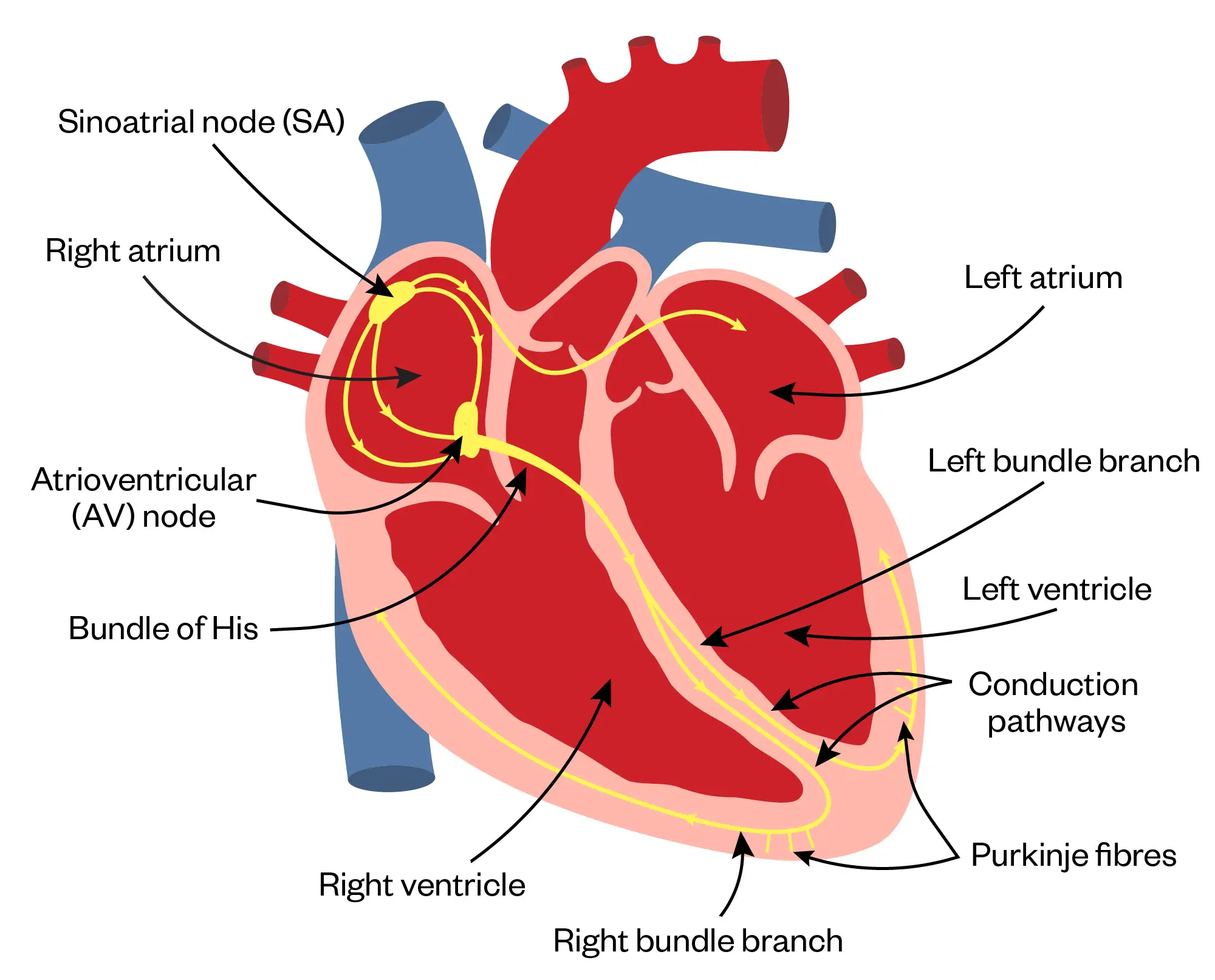
Shutterstock.com/The Pharmaceutical Journal
Re-entrant circuits
A core feature of the pathophysiology of SVT is the presence of re-entrant circuits. Re-entry requires the presence of a conduction pathway with different refractor periods, allowing unidirectional block and slow conduction. This results in the formation of a self-perpetuating loop (circuit) within the cardiac tissue, allowing repetitive activation and perpetuation of the arrythmia6. There are multiple factors that can lead to re-entrant tachycardia, including abnormal automaticity, triggered activity or a combination of both (see Box).
Box: Abnormal automaticity and triggered activity
Abnormal automaticity
The generation of spontaneous ectopic electrical impulses by the cells outside of the Sino-atrial node. In SVT, enhanced automaticity in atrial or AV nodal tissues can initiate and sustain the arrythmia. This enhanced automaticity may owe to altered ion channel function, changes in intracellular calcium handling and storage, or alterations in sympathetic or parasympathetic nervous system function7.
Triggered activity
Occurs when an action potential is elicited by after depolarisation, which can either be early (EAD) or delayed (DAD). EADs and DADs can result from disturbances in ion channel function, electrolyte imbalances or alterations in intracellular calcium levels. These abnormal depolarisations can trigger the onset of SVT by initiating a re-entrant circuit or causing premature (or ectopic) beats.
Junctional ectopic tachycardia
Junctional ectopic tachycardia (JET) is an arrhythmia often seen in infants post operatively, especially after cardiac surgery. It can have significant mortality if not promptly diagnosed and treated. JET originates in the AV node or AV junction, including the BH. It can be further classified into congenital junctional ectopic tachycardia (CJET) and postoperative junctional ectopic tachycardia (POJET)8.
Congenital junctional ectopic tachycardia usually occurs in infants aged under six months, but diagnosis can be delayed after this age too. Typically, infants with JET will have heart rates of approximately 200 to 250 beats per minute. Patients can also develop foetal tachycardia prenatally and present as congestive heart failure in the foetus. Postoperative JET usually presents within 72 hours after surgery. On physical examination, the patient is noted to have tachycardia with signs of congestive heart failure8,9.
The patient should be evaluated for electrolyte abnormalities and acidosis, along with a chest X-ray to evaluate for cardiomegaly and pulmonary oedema. An electrocardiogram should be checked to assess for narrow complex tachycardia.
Ventricular tachycardia
Ventricular tachycardia is a regular fast heartbeat that starts from the ventricles instead of starting from the SA node in the atria. Ventricular tachycardia can be described as sustained or non-sustained, and monomorphic or polymorphic10:
- Non-sustained VT lasts for at least three beats and terminates spontaneously (less than 30 seconds) and be asymptomatic;
- Sustained VT lasts for more than 30 seconds, or can require intervention if accompanied with haemodynamic instability. Sustained VT may cause symptoms such as dizziness, presyncope (feeling faint), syncope (fainting), breathlessness and may lead to cardiac arrest requiring medical intervention10;
- Monomorphic VT can be caused by a small area of abnormal electrical tissue or scarring. The beats can be visualised on an ECG and is seen as stable QRS morphology with no beat-to-beat variation as the impulses originate from the same point in the ventricles;
- Polymorphic VT can be caused by heart muscle disease, electrolyte imbalance, or channel ion disease. With polymorphic VT, there are differing QRS morphologies that can be visualised on an ECG, with beat-to-beat variation as the impulses originate from different points in the ventricles. Although the causes of polymorphic VT are more serious than other types of VT, they are also very rare and further investigations are needed to confirm this10.
Wolff-Parkinson-White syndrome
Wolff-Parkinson-White (WPW) syndrome is a congenital condition where an extra electrical pathway exists between the atria and ventricles, leading to abnormal heart rhythms. It is often diagnosed in childhood or adolescence.
It is characterised by the presence of an abnormal accessory pathway connecting the atria and ventricles. The accessory pathway allows electrical impulses to bypass the AV node, leading to rapid conduction and the formation of a re-entrant circuit. Pre excitation occurs when the accessory pathway conducts the impulse to the ventricles in antegrade, this results in a shortened PR interval and the presence of a delta wave on an ECG11 (see Figure 2). The presence of a delta wave on an ECG subsequently causes a broadened QRS11.
Long QT syndrome
Long QT syndrome (LQTS) is characterised by a prolonged QT interval on an ECG, which may be congenital or acquired12. The QT interval is a measurement made on an ECG, which is used to assess the electrical properties within the heart. This interval is calculated as the time from the start of the Q wave to the end of the T wave. This overall approximates the time taken from when the cardiac ventricles start to contract to when they finish relaxing.
In congenital LQTS, mutations within 16 genes can result in a variety of dysfunction channelopathies affecting myocardial repolarisation, thus prolonging the QT interval (see Figure 3). It usually affects children and young adults, and can occur in up to 1 in 2,000 people. In 70% of people with LQTS, gene testing can help identify which ion channels are involved13.
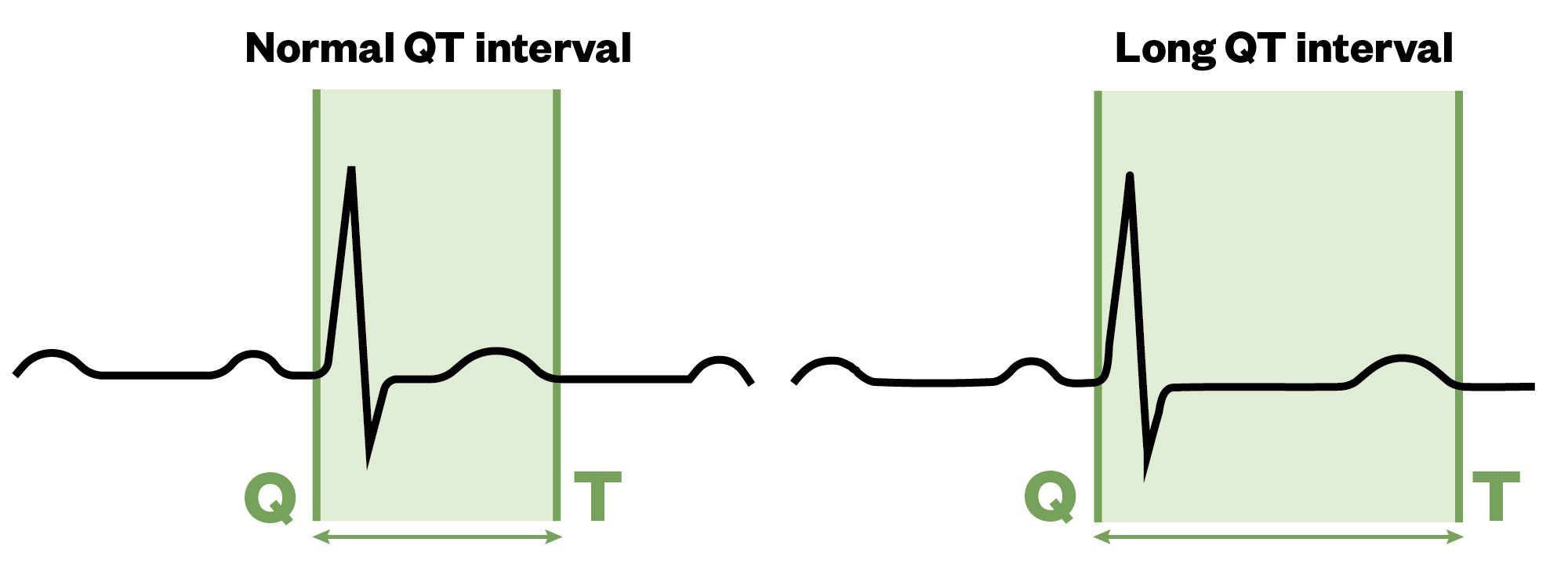
The Pharmaceutical Journal
For the most part, two of the potassium channels that regulate the movement of potassium ions from the inside to the outside of the cell are affected; however, in a very small group of patients with LQTS, a sodium channel that regulates the flow of sodium ions from the outside to the inside of cells is affected12,13.
A normal QTc interval does not exclude the presence of a long QT syndrome. Up to 40% of people with genetic mutations can have a QTc interval within the normal limits. The QT interval varies for males and females and with age. Therefore, a specific figure is subjective to the clinician’s experience. Generally, practice within paediatric establishments will investigate intervals equal to or more than 450ms. Any QTc interval equal or more than 480ms however will need great study and inspections12,13.
Many children have no symptoms, but fainting is common in those who do. It may also cause cardiac arrest. In many cases, young people may present with cardiac arrest or unexplained syncope which can often be misdiagnosed as epilepsy. Cardiac syncope is characterised with premonitory symptoms, such as palpitations and chest pain. During these episodes, patients will often show signs of cyanosis and changes in pallor13.
It is to be noted that resources, such as crediblemeds.org, can be a good reference for healthcare professionals who see patients with LQTS to determine which drugs are safe to use.
Brugada syndrome
Brugada syndrome (BrS) is an autosomal dominant inherited channelopathy. It is associated with a typical pattern of ST-segment elevation and potentially lethal ventricular arrhythmias in otherwise healthy subjects.
Although it typically presents in young adults, it is also known to present in children and infants, especially in the presence of fever. Since ECG findings are a requisite for the diagnosis of BrS, it is hard to know the true prevalence of BrS as presumably many patients are asymptomatic[14]. BrS is thought to be responsible for 4–20% of sudden unexplained deaths in the young. Also, 10–20% of sudden infant death syndrome owes to identifiable inherited channelopathies, including BrS14,15.
The most common primary presentation of BrS in children is known family history, followed by incidental findings on an ECG. Other symptoms include syncope and incidental findings of murmurs. In less than 1% of patients, sudden death is a rare occurrence and posthumously BrS is diagnosed. This reflects the general proportion of presentation in adult studies.
Most syncopal episodes occur at rest and are often precipitated by fever, including vaccination-related fever episodes. Palpitations related to atrial fibrillation (AFib) may be the initial presentation in some cases as well14–16.
It is important to distinguish patients with BrS from those with a Brugada like ECG pattern. The Brugada pattern can also be induced by medications and toxins, including sodium channel blockers, beta-blockers, tricyclic antidepressants, local anaesthetics, alcohol and cocaine. The clinical significance of Brugada pattern in the absence of symptoms or family history of BrS is undetermined. The diagnosis is based on a combination of clinical symptoms, positive family history and specific ECG findings that are either present regularly on a 12 lead ECG at rest or provoked by the use of agents such as ajmaline. In addition, genetic testing is usually carried out to confirm the mutation SCN5A as another form of diagnosing BrS14–16.
Diagnosis of arrhythmias
Diagnosing any type of paediatric arrhythmias requires a combination of clinical evaluation, medical history and various diagnostic tests, including but not limited to:
- Electrocardiogram — records the electrical activity of the heart and is a fundamental tool for diagnosing arrhythmias;
- Holter monitor — a portable ECG device worn for an extended period to capture intermittent arrhythmias;
- Echocardiogram — an ultrasound of the heart that helps identify structural heart defects;
- Exercise stress test — monitors heart rhythms during physical activity to detect stress-induced arrhythmias.
Treatment options
The treatment of paediatric arrhythmias depends on the type, severity, and underlying cause of the arrhythmia. Treatment will usually involve one or more of the following:
Medications
Anti-arrhythmic drugs can help regulate heart rate and rhythm (see Figure 4 and Table).
Cardioversion
This is a way of restoring sinus rhythm. This can be achieved with drugs (pharmacological) or electrical shocks (DCCV).
Ablation
A procedure called an electrophysiology study (EP) and ablation may be suggested. This is a minimally invasive procedure to destroy or isolate abnormal electrical pathways. The doctor will use either cryoablation (freezing therapy) or radio frequency ablation (heating therapy) on the affected area, which should stop the abnormal signals.
Ablation works by using a targeted beam of energy to destroy the tissues causing the abnormal signals. This in turn helps regulate the AV node to pace the patient. Some children may require pacemakers or implantable cardioverter-defibrillators (ICDs) to manage severe arrhythmias if there is a risk of sudden collapse. The ICD is inserted in the wall of the chest and will continually monitor the heart rhythm and deliver a life-saving shock if it detects an abnormal rhythm17.
Pharmacological management
Most antiarrhythmic medications are categorised depending on where they act within the cardiac action potential based on the Vaughan Williams classification system18 (See Figure 419–26).
The sharp rise in voltage — 0 — corresponds to the influx of sodium ions, whereas the two decays — 1 and 3 — correspond to the sodium-channel inactivation and the repolarizing efflux of potassium ions. The characteristic plateau — 2 — results from the opening of voltage-sensitive calcium channels. Other additional agents including adenosine and digoxin are used that have alternative mechanisms18.
Patient counselling points
Every antiarrhythmic comes with its own set of counselling points and similarly to adults it is always ideal to consult the product specification and BNF/BNFc where appropriate. In addition to this, the Medicines for Children website has a great number of patient information leaflets that can also be used as reference when counselling.
It is to be noted that many of these anti-arrhythmic medicines come in different strengths, therefore a counselling element is to ensure parents are aware that the volume of a dose may change if a different strength is supplied. Below is a very brief table on the basic points of counselling to consider2,3,19–29.
Best practice
- Exploring the pathophysiology and underlying mechanisms that contribute to the development of arrhythmias in children can often provide the appropriate appreciation and understanding of the mechanism of treatment and choice of management;
- Children are not small adults: they metabolise drugs differently and, in some cases, they need larger doses than you would expect. It is important to consider the age of the child when looking at interactions, CYP450 enzymes do not mature until they are young adults. For example, it may not absolutely necessary to consider enzyme induction/inhibition in a 3-year-old patient but keeping this information as part of risk assessing the overall treatment plan;
- Ensure that parents and carers are thoroughly counselled about the formulation and dosing for any medications prescribed (e.g. explain that digoxin oral solution should not be diluted);
- Children can be amazingly accurate sources of information, but unfortunately they are also quite easily influenced. It can be difficult in these circumstances to know if a patient is experiencing a side effect; therefore, it may be ideal to also to look for more objective signs, such as greying of the skin with amiodarone (noting that adverse effects are more likely to occur during the first week of treatment), breathlessness on rest, etc.;
- Making a medication chart for the child — which also helps the parents remember when doses are due — allows for the child to feel in control of their treatment. In addition to this, it allows for the foundation to be set when children are transitioning to adult services;
- Engaging with the child can aid in compliance and improves outcomes into adulthood. Educating the patient and discussing the medication on a one-to-one basis can better improve adherence.
Useful resources
- Brugadadrugs.org: a non-profit initiative developed as an aid to physicians who treat patients with Brugada syndrome and as an aid to patients with Brugada syndrome and their families providing up to date information on safe drug use in Brugada syndrome;
- https://www.medicinesforchildren.org.uk: a partnership programme of Royal College of Paediatrics and Child Health (RCPCH), Neonatal and Paediatric Pharmacists Group (NPPG) and WellChild, offering medicines information pages covering many of the drugs that are prescribed and recommended for children by healthcare professionals;
- Re-entry circuits: a brief explanation of the physiological basis of re-entry.
- 1.Yap NYS, Yue A, Sadagopan S, Hayes N. Practical approach to tachyarrhythmia in children. Paediatrics and Child Health. 2022;32(12):448-462. doi:10.1016/j.paed.2022.10.002
- 2.Supraventricular tachycardia . Great Ormond Street Hospital For Children NHS Foundation Trust. Published December 2020. Accessed July 2024. https://www.gosh.nhs.uk/conditions-and-treatments/conditions-we-treat/supraventricular-tachycardia
- 3.Ahmad M, Reddy S, Barkhane Z, Elmadi J, Satish Kumar L, Pugalenthi LS. Hyperthyroidism and the Risk of Cardiac Arrhythmias: A Narrative Review. Cureus. Published online April 22, 2022. doi:10.7759/cureus.24378
- 4.Josephson ME, Wellens HJJ. Differential Diagnosis of Supraventricular Tachycardia. Cardiology Clinics. 1990;8(3):411-442. doi:10.1016/s0733-8651(18)30348-5
- 5.Desai D, Hajouli S. Treasure Island. StatPearls. Published January 2024. Accessed July 2024. https://www.ncbi.nlm.nih.gov/books/NBK558923/
- 6.Issa ZF, Miller JM, Zipes DP. Electrophysiological Mechanisms of Cardiac Arrhythmias. Clinical Arrhythmology and Electrophysiology. Published online 2019:51-80. doi:10.1016/b978-0-323-52356-1.00003-7
- 7.Ashraf M, Goyal A. Junctional ectopic tachycardia. StatPearls. Published April 2023. Accessed July 2024. https://www.ncbi.nlm.nih.gov/books/NBK560851
- 8.Kylat RI, Samson RA. Junctional ectopic tachycardia in infants and children. Journal of Arrhythmia. 2019;36(1):59-66. doi:10.1002/joa3.12282
- 9.Kafali HC, Ergul Y. Common Supraventricular and Ventricular Arrhythmias in Children. Turk Arch Pediatrics. 2022;57(5):476-488. doi:10.5152/turkarchpediatr.2022.22099
- 10.Roston TM, Vinocur JM, Maginot KR, et al. Catecholaminergic Polymorphic Ventricular Tachycardia in Children. Circ: Arrhythmia and Electrophysiology. 2015;8(3):633-642. doi:10.1161/circep.114.002217
- 11.Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: A long-term follow-up of 22 years. American Heart Journal. 2001;142(3):530-536. doi:10.1067/mhj.2001.117779
- 12.Long QT syndrome. BMJ Best Practice. Published 2023. Accessed July 2024. https://bestpractice.bmj.com/topics/en-gb/829
- 13.Long QT syndrome (LQTS) – cardiac risk in the young . Cardiac Risk in the Young. Published 2023. Accessed July 2024. https://www.c-r-y.org.uk/long-qt-syndrome
- 14.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. Journal of the American College of Cardiology. 1992;20(6):1391-1396. doi:10.1016/0735-1097(92)90253-j
- 15.Wilde AAM, Antzelevitch C, Borggrefe M, et al. Proposed Diagnostic Criteria for the Brugada Syndrome. Circulation. 2002;106(19):2514-2519. doi:10.1161/01.cir.0000034169.45752.4a
- 16.Michowitz Y, Milman A, Andorin A, et al. Characterization and Management of Arrhythmic Events in Young Patients With Brugada Syndrome. Journal of the American College of Cardiology. 2019;73(14):1756-1765. doi:10.1016/j.jacc.2019.01.048
- 17.Griksaitis MJ, Rosengarten JA, Gnanapragasam JP, Haw MP, Morgan JM. Implantable cardioverter defibrillator therapy in paediatric practice: a single-centre UK experience with focus on subcutaneous defibrillation. EP Europace. 2013;15(4):523-530. doi:10.1093/europace/eus388
- 18.Barton AK, McGowan M, Smyth A, Wright GA, Gardner RS. Classification and choice of antiarrhythmic therapies. Prescriber. 2020;31(3):11-17. doi:10.1002/psb.1828
- 19.Adenosine. Paediatric Formulary Committee. BNF for Children . Accessed July 2024. https://bnfc.nice.org.uk/drugs/adenosine/#indications-and-dose
- 20.Sotalol. Paediatric Formulary Committee. BNF for Children . Accessed July 2024. https://www.medicinescomplete.com/#/content/bnfc/_514586448?hspl=sotalol
- 21.Flecainide. Paediatric Formulary Committee. BNF for Children . Accessed July 2024. https://www.medicinescomplete.com/log-in/#/content/bnfc/_264345609?hspl=flecainide
- 22.Andrikopoulos GK. Flecainide: Current status and perspectives in arrhythmia management. WJC. 2015;7(2):76. doi:10.4330/wjc.v7.i2.76
- 23.Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. The American Journal of Cardiology. 1999;84(9):20-28. doi:10.1016/s0002-9149(99)00698-0
- 24.Handbook of Emergency Cardiac Care. AHA; 2006.
- 25.Fitton A, Sorkin EM. Sotalol. Drugs. 1993;46(4):678-719. doi:10.2165/00003495-199346040-00007
- 26.Amiodarone. Paediatric Formulary Committee. BNF for Children . Accessed July 2024. https://www.medicinescomplete.com/log-in/#/content/bnfc/_675219041?hspl=amiodarone
- 27.Propranolol. Paediatric Formulary Committee. BNF for Children . Accessed July 2024. https://www.medicinescomplete.com/#/content/bnfc/_202997782?hspl=propranolol
- 28.Rankin AC, Brooks R, Ruskin JN, McGovern BA. Adenosine and the treatment of supraventricular tachycardia. The American Journal of Medicine. 1992;92(6):655-664. doi:10.1016/0002-9343(92)90784-9
- 29.Digoxin. British National Formulary Joint Formulary Committee. Accessed July 2024. https://bnfc.nice.org.uk/drugs/digoxin