View the full infographic here.
Discovery and development of the pill
Progesterone was first synthesised in the 1950s and was licensed as an oral contraceptive in 1960
1951: Carl Djerassi, a chemist at pharmaceutical company Syntex, creates a progesterone pill by synthesising hormones from yams
1952: Chemist Frank Colton at the pharmaceutical company Searle also develops a synthetic progesterone pill
1954: Biologist Gregory Pincus and gynecologist John Rock conduct the first human trials on 50 women in the US. The pill is contaminated with oestrogen during synthesis but purifying it leads to breakthrough bleeding so it is retained
1956: After large clinical trials in Puerto Rico, the pill is found to be 100% effective
1957: Enovid 10mg (9.85mg norethynodrel and 150µg mestranol; Searle) is approved by the US Food and Drug Administration (FDA) for “menstrual irregularities”. It is released onto the British market as Enavid
1960: The FDA approves contraception as an additional indication for Enovid 10mg
1961: Conovid 5mg is approved by the British Family Planning Association. Serious concerns are raised after three fatal cases of blood clots in women taking COCs
1967: Epidemiological studies link the pill with thrombosis
1970s: Second generation pills are launched, which contain lower amounts of hormones
1974: Free contraception is introduced in the UK leading to increased uptake
1980s: Third generation pills are launched to reduce androgenic and metabolic side effects
1995: The UK Committee on Safety of Medicines says third generation COCs should not be used first line because of the risk of blood clots. This “pill scare” is thought to lead to an increase of 9% in the abortion rate in 1996
1999: The Medicines Commission says third generation pills can be prescribed first line after all
2001: The EMA reviews third generation COCs and confirms a small increased risk of VTE compared with second generation pills. Fourth generation COCs are released
2009: The first COC containing the oestrogen estrodiol is launched
2013: The EMA reviews third and fourth generation COCs and concludes that their benefits continue to outweigh their risks, and the well-known risk of VTE with all COCs is small
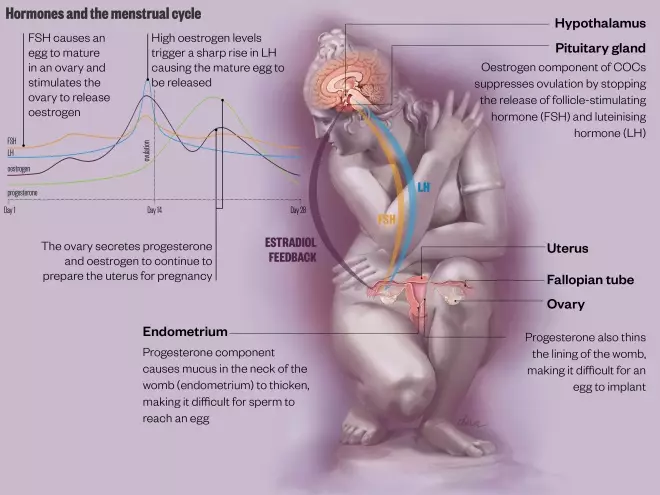
How combined oral contraceptives work
Combined oral contraceptives (COCs) contain synthetic versions of the sex hormones oestrogen and progesterone, steady levels of which convince the pituitary gland of a pregnancy.
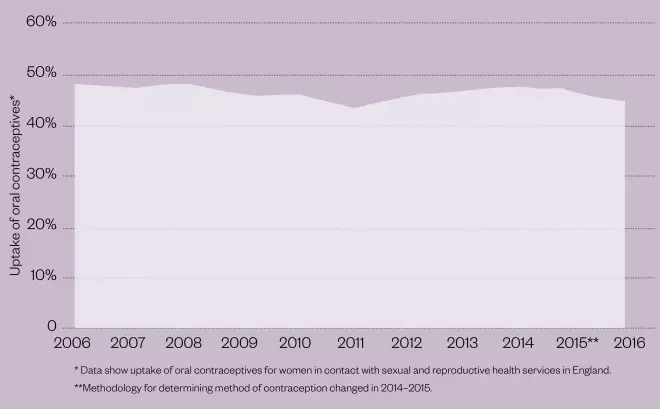
The enduring popularity of the pill
There has been little change in the proportion of women using oral contraceptives over the past ten years.
Contraindications and interactions
Women should not take the combined oral contraceptive pill if they:
- are pregnant
- smoke and are 35 or older
- stopped smoking less than a year ago and are 35 or older
- are very overweight
Or if they have or have had:
- a blood clot
- a heart abnormality or heart disease, including high blood pressure
- severe migraines, especially with aura (warning symptoms)
- breast cancer
- disease of the gallbladder or liver
- diabetes with complications or diabetes for the past 20 years
The following medicines can interact with combined oral contraceptive pills:
- antibiotics (rifabutin, rifampicin)
- antiepileptics (carbamazepine, eslicarbazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, rufinamide, topiramate)
- antiretrovirals (ritonavir, ritonavir-boosted protease inhibitors, efavirenz, nevirapine)
- antidepressants (St John’s wort)
- others (modafinil, bosentan, aprepitant)
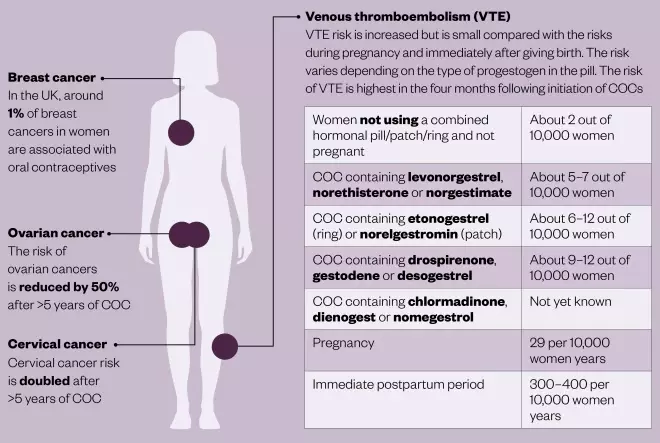
Risks with taking combined oral contraceptives
COCs increase the risk of blood clots and some cancers, but reduce the risk of other cancers.
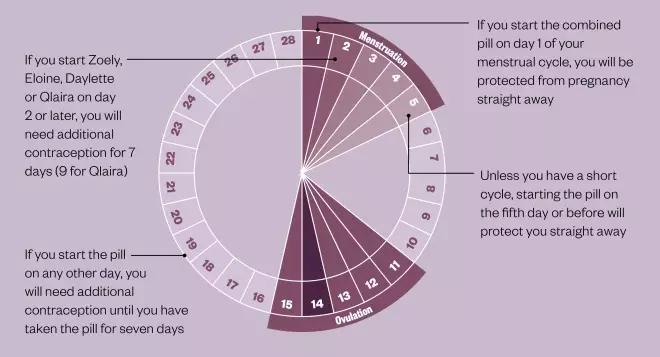
Types of pills
There are two main types of COCs: monophasic (contain the same amount of hormones in each pill) and phasic (contain different amounts of hormones).
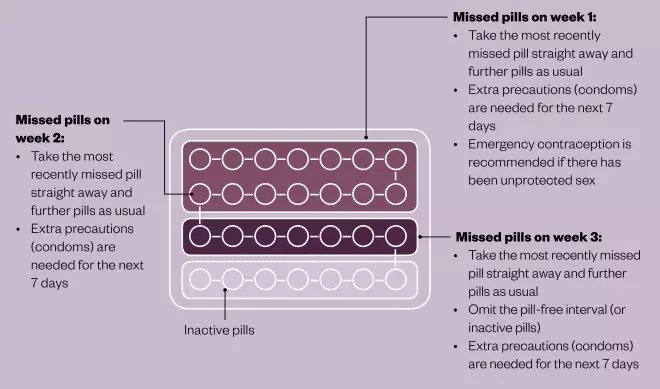
Missed pill advice
If only one active pill is missed, take the missed pill straight away and further pills as usual. No extra precautions are needed. If more than one pill is missed, follow advice above. This advice doesn’t apply to Qlaira, Zoely, Eloine and Daylette — consult product literature.
| Type of COC | Brand name | Oestrogen | Progestogen |
|---|---|---|---|
| Sources: BNF, MIMS | |||
| Monophasic 21-day | Bimizza Gedarel 20/150 Lestramyl 20/150 Mercilon Munalea 20/150 | Ethinylestradiol 20mcg | Desogestrel 150mcg |
| Aidulan 20/75 Femodette Millinette 20/75 Sunya 20/75 | Ethinylestradiol 20mcg | Gestodene 75mcg | |
| Loestrin 20 | Ethinylestradiol 20mcg | Norethisterone acetate 1mg | |
| Cimizt Gedarel 30/150 Lestramyl 30/150 Marvelon Munalea 30/150 | Ethinylestradiol 30mcg | Desogestrel 150mcg | |
| Acondro Dretine Lucette Yacella Yasmin Yiznell | Ethinylestradiol 30mcg | Drospirenone 3mg | |
| Femodene Katya 30/75 Millinette 30/75 Aidulan 30/75 | Ethinylestradiol 30mcg | Gestodene 75mcg | |
| Elevin Levest Maexeni Microgynon 30 Ovranette Rigevidon | Ethinylestradiol 30mcg | Levonorgestrel 150mcg | |
| Loestrin 30 | Ethinylestradiol 30mcg | Norethisterone acetate 1.5mg | |
| Cilest Cilique Lizinna | Ethinylestradiol 35mcg | Norgestimate 250mcg | |
| Brevinor | Ethinylestradiol 35mcg | Norethisterone 500mcg | |
| Norimin | Ethinylestradiol 35mg | Norethisterone 1mg | |
| Norinyl-1 | Mestranol 50mcg | Norethisterone 1mg | |
| Monophasic 28-day | Femodene ED | Ethinylestradiol 30 mcg | Gestodene 75mcg |
| Microgynon ED | Ethinylestradiol 30 mcg | Levonorgestrel 150mcg | |
| Zoely | Estradiol (as hemihydrate) 1.5mg | Nomegestrol acetate 2.5mg | |
| Eloine | Ethinylestradiol 20mcg | Drospirenone 3mg | |
| Daylette | Ethinylestradiol 20mcg | Drospirenone 3mg | |
| Phasic 21-day | Triadene | Ethinylestradiol 30/40/30mcg | Gestodene 50/70/100mcg |
| Logynon TriRegol | Ethinylestradiol 30/40/30mcg | Levonorgestrel 50/75/125mcg | |
| Synphase | Ethinylestradiol 35mcg | Norethisterone 500mcg/1mg/500mcg | |
| Phasic 28-day | Logynon ED | Ethinylestradiol 30/40/30mcg | Levonorgestrel 50/75/125mcg |
| Qlaira | Estradiol valerate 3/2/1mg | Dienogest 2/3mg | |
How to have effective consultations on contraception in pharmacy
What benefits do long-acting reversible contraceptives offer compared with other available methods?
Community pharmacists can use this summary of the available devices to address misconceptions & provide effective counselling.
Content supported by Bayer
References
Editorial adviser: Nuttan Tanna, pharmacist consultant, women’s health and older people, London North West Healthcare NHS Trust, Harrow, London
Sources: British National Formulary, NHS Digital, European Medicines Agency, Medicines and Healthcare products Regulatory Agency, Faculty of Sexual and Reproductive Healthcare, MIMS.
Illustration: Alex Baker
Infographic: Maria Gonzalez

