Mclean/Shutterstock.com
After reading this article, you should be able to:
- Understand the principles of effective inhaler prescription;
- Ensure a patient-centred approach that optimises both disease outcome and the requirement to reduce the environmental impact of prescribing;
- Be able to conduct a medication review for patients using inhalers.
Introduction
In October 2020, the NHS became the world’s first health service to commit to reaching ‘net zero’ emissions by 2045[1]. The NHS is responsible for 4% of England’s total carbon footprint, of which medicines account for 25% of emissions — inhalers account for 3% of these emissions[1].
Propellant gases within metered dose inhalers (pMDI) are responsible for most of these emissions[2]. The ‘NHS Long-Term Plan’, published in 2019, aims to reduce pMDI usage by 50% by 2030 by increasing the use of lower carbon devices, such as dry powder inhalers (DPI) and soft mist inhalers (SMI), where appropriate[2].
The 2022/2023 NHS England Impact and Investment Fund (IIF) prioritises activity for primary care networks (PCNs), including optimising respiratory medicine use and supporting the reduction in carbon emissions[3]. The IIF incentivises PCNs to reduce the volume of pMDIs prescribed as a percentage of all non-salbutamol inhalers and to reduce carbon emissions per salbutamol inhaler prescribed by using DPIs and low-volume salbutamol pMDIs[3].
While most of the carbon impact from inhalers comes from the hydrofluoroalkane (HFA) propellants currently used in pMDIs (HFA227 and HFA134), environmental impacts also arise during the manufacturing process and end-of-life waste disposal[4]. This affects all inhalers including DPIs, SMIs and pMDIs[5]. Novel HFA propellants such as HFA152 with low global warming potential are expected to be available in 2025. The use of plastics further contributes to other environmental harms, including fossil fuel depletion, marine eutrophication and freshwater and marine ecotoxicity, and these costs are significantly greater with DPIs than with pMDIs[5].
In England, pMDIs account for around 75% of all inhaler prescriptions and it is estimated that they are responsible for releasing the equivalent of 635,000 metric tons of CO2 each year[6]. Therefore, if 10% of patients switched to DPIs, there would be a reduction of 58,000 metric tons of CO2, equivalent to 180,000 cars making a round-trip between London and Edinburgh[7,8]. This is thought to be achievable because other European countries prescribe far fewer pMDIs as a proportion of all types of inhalation device; for example, pMDIs account for only 10–20% of all devices used across Scandinavia[9].
As with every consumable resource, there is an environmental cost to all types of inhaler and it is important that prescribing decisions are made that maximise individual patient clinical benefit while considering ways to safely minimise the environmental impact[10].
The IIF addresses optimising respiratory medicine use by focusing on low prescribing rates of inhaled corticosteroid inhalers (ICS) in England (the most effective drugs for preventing asthma exacerbations), and reducing excessive short-acting beta2-agonist (SABA) usage, which is associated with higher exacerbation rates and mortality[3,11,12]. The IIF also encourages PCNs to ensure asthma patients prescribed ICS are using them regularly to decrease SABA use[3].
The IIF highlights the importance of shared decision-making with patients and the need to undertake a full assessment of inhaler technique prior to any changes being made. However, concerns have been raised about how the IIF targets might be implemented, including reports of ‘blanket switching’[13]. Wholesale switches across a patient cohort may reduce the carbon impact on paper but risks loss of disease control and increased exacerbation rates that could quickly offset any initial CO2 savings. Additionally, acute medical care has a carbon impact of 125kg of CO2 equivalent per day, compared with 10–28kg of CO2 equivalent per pMDI, and so poorly targeted switches that result in worse patient outcomes and hospitalisation may inadvertently lead to higher carbon emissions for the NHS than continuing pMDIs[14].
Principles of effective inhaler prescribing
While there are incentives to switch patients from pMDIs to lower carbon devices (e.g. DPIs and SMIs) it is important to note that, above all, the priority for healthcare professionals is to make the best decision for each patient to improve their health. However, there are opportunities to change treatment for individuals and cohorts of patients when treatment has not been optimised. This may result in the dual benefit of improving disease control alongside reducing the carbon impact of inhaler prescribing.
Prioritise patient groups who may benefit from switching inhalers
There are several patient groups who may be suitable for review and potentially switching inhaler devices and individual patients should be prioritised for review rather than attempting a blanket inhaler switch policy. This includes those with poor disease control, poor inhaler technique, poor adherence, excessive SABA use and those prescribed mixed types of inhaler device.
Patients with poor disease control or frequent exacerbations of asthma or COPD are likely to be frequent SABA users and have higher levels of unplanned care, potentially resulting in A&E attendance and hospital admission, both of which have a high carbon impact[12]. These patients should have an urgent review of diagnosis and their treatment optimised. Similarly, patients with asthma who have poor adherence to maintenance inhalers and people using more than three SABA inhalers per year are more likely to have poor disease control and higher rates of severe exacerbations and should also be prioritised for review[12,15]. It is for this reason that the IIF incentives PCNs to optimise respiratory medicine use[3].
Patients prescribed mixed types of inhaler devices should also be prioritised for review to assess whether it is possible to safely rationalise their prescription. Disease control can be affected when patients are prescribed a mixture of devices that require different inhalation techniques (e.g. using a DPI maintenance inhaler with a pMDI reliever). This practice is associated with worse disease control and more exacerbations compared with patients using the same or similar inhaler devices, likely caused by the variation in inhalation technique, leading to errors[16,17].
In each case, inhaler technique should be assessed to determine the most appropriate inhaler device for the patient. By ensuring that each patient can use their inhaler device correctly, asthma and COPD control can be improved, with reductions in excessive SABA use, exacerbations and hospitalisations); CO2 emissions from inhaler use should then fall too[18,19].
Structure the patient medication review to ensure shared decision-making
A review should start by assessing the patient’s current devices and technique to determine whether a patient can use their existing inhaler device(s) optimally. If they can, then there may be no reason to change this device and other interventions should be explored in order to improve disease control (e.g. addressing poor adherence, education and lifestyle management).
If a new inhaler device is needed, prescribing decisions should be individualised and an appropriate device chosen for everyone. The Assess, Choose, and Train (ACT) decision tool can be used to structure the review to ensure shared decision-making (see Figure 1)[20]:
- Assess: Patients should be assessed on their inspiratory flow in order to determine whether they are physically able to inhale through DPIs or aerosol devices in an effective manner;
- Choose: An appropriate device should be chosen, which may depend on local formulary, cost and environmental considerations;
- Train: the patient should be taught how to use the device using the seven steps required for good inhaler technique.
Teaching and checking inhaler technique is central to the consultation, as poor inhaler technique results in worse disease control and patient outcomes[21,22]. Misuse or misunderstanding of inhalers can contribute to asthma deaths and inadequate inhaler technique can also lower drug deposition in the lungs, leading to medication wastage, poor symptom control and lower quality of life[23].
More information on the assessment of inhaler technique and how to select the right inhaler can be found below.
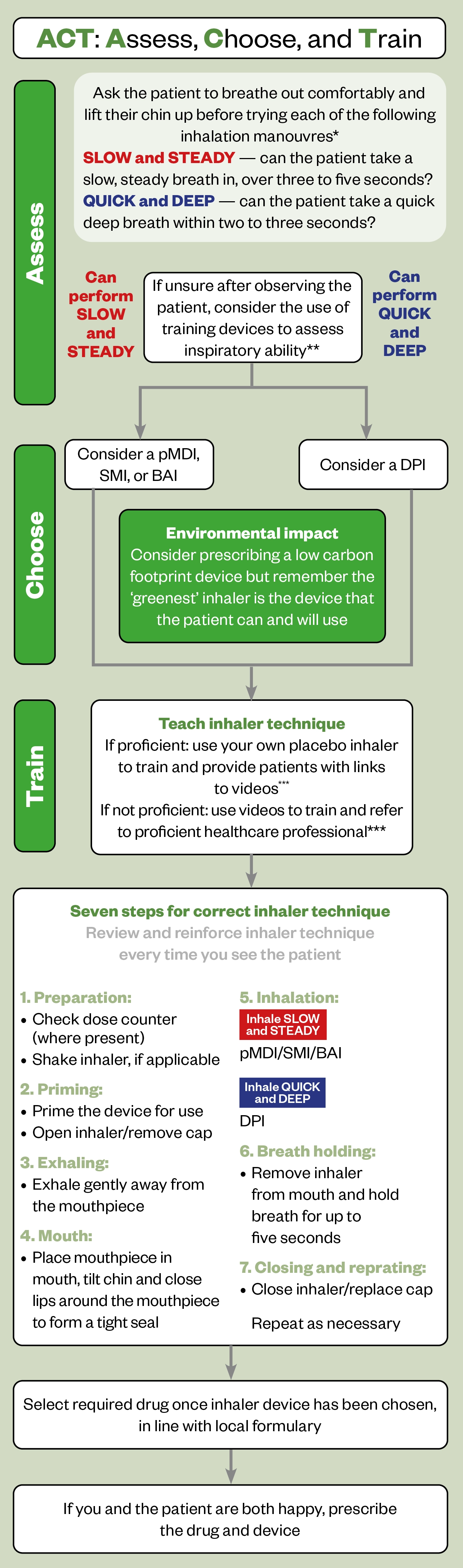
* If the patient can perform both inhalation manoeuvres, choose according to patient preference
** Examples of training devices that can be used to assess inspiratory ability are: AIM machine, Clip-Tone, Flo-Tone, In-Check DIAL inspiratory flow meter, placebo whistles
*** Training videos developed by the UK Inhaler Group can be found on the Asthma UK website and RightBreathe
BAI: breath-actuated inhaler; DPI: dry powder inhaler; pMDI: pressurised metered dose inhaler; SMI: soft mist inhaler
It is important to prepare and engage patients in the decision-making process to ensure a personalised approach. Patient reviews are required to identify the type of inhaler that best fits their inspiratory flow and accounts for their preferences. The ‘no decision about me without me’ approach should be used, rather than a blanket switch to a particular device[24]. Problems with inhaler switching may arise when patients have had no time to prepare or engage in the decision-making process or were not previously aware of any planned changes. Lack of inhaler technique education could result in the inability to use the device correctly or disengaging with therapy leading to worsening disease control[25]. In these cases, if the patient’s condition deteriorates, the cost savings and carbon reduction benefits of switching is lost.
Select the right inhaler type or device
When it comes to effective switching, there are several important considerations for healthcare professionals when selecting the most appropriate inhaler type. These include:
Understanding the science behind inhaled drug delivery to ensure the inhaler device(s) selected are effective
Drugs within inhalers are formulated to provide the optimal particle size of 1–5 micrometre to be deposited in the peripheral airways; those that are too small may be exhaled and those that are too large will impact in the oropharynx and large conducting airways[26].
Checking the patient’s inspiratory flow rate to determine the appropriate device
Many people with asthma and COPD inhale too fast using pMDIs, resulting in suboptimal response to treatment[27,28]. When using an aerosol inhaler, such as a pMDI or SMI, patients should be advised to inhale slowly and steadily over three to five seconds, as the aerosol is generated by the inhaler device and has sufficient speed to penetrate the peripheral airways[20]. If patients inhale quickly and deeply with this device, the aerosol is accelerated, impaction occurs in the oropharynx and the drug does not reach the peripheral airways, resulting in loss of effect, and increased risk of adverse drug reactions (ADRs)[19]. In one study, increasing the speed of inhalation from 30L/min (slow) to 180L/min (fast) resulted in a 30% reduction in whole-lung deposition. The use of a spacer will increase drug delivery of pMDI drugs; they should be prescribed for most patients and replaced every 6–12 months[29].
When using a DPI patients should be advised that quick and deep inhalation within two to three seconds is required[20]. This immediate and quick inhalation rate against the internal resistance of the inhaler device generates a large internal turbulent force in the DPI device, which produces particles of a size distribution that will penetrate the peripheral airways[26,30]. Inhaling too slowly results in inhalation of large particles, which will impact in the oropharynx resulting in loss of effect and increase the risk of ADRs.
If the patient is capable of both types of inspiratory manoeuvre (quick and deep + slow and steady), choose the type of inhaler device that the patient prefers and requires their natural inspiratory manoeuvre. Remind patients to take a full, deep breath through all devices. This allows the aerosol to penetrate peripheral bronchioles and breath-holding allows gravitational sedimentation to increase drug deposition in the lungs[26].
A DPI or SMI should only be prescribed as the first choice if clinically appropriate. Patients should not be switched without checking inhaler technique or patient preference. There will be some patients where it will be appropriate to continue to use a pMDI, such as small children, frail and older people. Switching to a DPI purely for environmental reasons may not be appropriate.
If a patient is using a preventer inhaler, check that they are using it regularly and not requiring their reliever (SABA) inhaler excessively. Use of the reliever on more than three days a week may indicate inadequate disease control and a full review of treatment, including review of inhaler technique, would be appropriate[31].
Ensure treatment decisions are underpinned by guidance
Asthma and COPD management should be optimised with patients on the correct treatment in line with national guidelines[31,32].
Prescribe smartly
Once a type of inhaler has been chosen, a brand and regimen should be considered to minimise the carbon footprint. The Greener Practice guide includes tables of inhalers by carbon footprint category, aimed to help inform alternative inhaler choices[33].
If a patient is concerned about the environmental impact of their inhalers but is unable to change away from a pMDI, they should be reassured that the greenest inhaler someone can use is one that they can use effectively to have good disease control, minimise the use of reliever inhalers and avoid hospitalisation[4]. No patient should feel stigmatised about their inhaler use and carbon footprint.
Check inhaler technique and provide education and support to optimise medicines use
The National Institute for Health and Care Excellence (NICE) and the British Thoracic Society recommend that patients receive training on the use of a new inhaler device prior to prescribing and have their technique assessed at each clinic visit[31,32]. Despite this, some research has showed that up to a quarter of patients do not receive any verbal instruction on how to use their inhaler device, with only 11% of patients were followed up at reviews on their technique[23,34].
Patient education on how to use inhalers has shown to improve asthma symptoms, improve forced expiratory volume (FEV1) values and reduce SABA usage over time[35]. The method of inhaler technique training is important, because demonstrating correct inhaler technique is more likely to achieve optimal inhaler technique compared to verbal instruction or provision of a patient information leaflet[36].
Correct inhaler technique should be demonstrated and observed in use before it is prescribed. It may be useful to share links to videos, including those from Asthma+Lung UK, to help reinforce the correct technique; this is an adjunct, not a replacement to teaching and checking.
Reassure and counsel patients to agree plans for follow-up and assessment
There are several important considerations and counselling points relating to switching inhalers and long-term assessment and management that pharmacists should be aware of, these include:
- Reassuring patients that if they do change to a lower carbon impact inhaler but feel that their disease control worsens then they can switch back again[33];
- Encouraging patients to return all used/unwanted inhalers to community pharmacies for disposal. Inhalers should not be placed in household waste as this allows the release of HFAs into the environment. Pharmacies may have access to a recycling scheme or alternatively the inhalers are sent to be incinerated, which degrades the HFAs and lowers the environmental impact[37]. Patients should also be shown how to identify when an inhaler is fully empty and to use it until it is empty before disposing of it[4];
- Inhaler technique should be reinforced, regularly assessed and corrected where necessary[31,32]. Studies have shown that inhaler technique can deteriorate within two to three months of prescribing and so should ideally be rechecked and reinforced within this timeframe[38];
- A follow-up appointment should be arranged to monitor response to treatment and to ensure patients are happy with the change. The timeframe for the follow-up appointment should be judged based on person-centred considerations and the level of support that the patient may require. A trial of treatment changes in asthma may last for weeks or even months[31]. Consider collaborative approaches across general practice, hospital and community pharmacy to ensure patients are supported in treatment changes. Community pharmacists are well placed to support patients and prescribers should be encouraged to refer patients to their pharmacy when new medicines are commenced for people with asthma and COPD.
Summary
Although the environmental impact of inhalers needs to be reduced, this must be carried out without causing harm to patients. The priority should be to optimise disease control using the guidelines available that will reduce the excess use of SABA inhalers. Inhalers should be changed to lower carbon devices only in discussion with patients and consider their preference, inhaler technique and inspiratory flow. It is important to remember that the greenest Inhaler is the inhaler device that the patient can use and will use[19].
Financial and conflicts of interest disclosures
T.G.D.C. has received sponsorship to attend conferences from Napp and their employer has received payment for consulting and lecturing from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Insmed and Novartis. N.A. has received sponsorship to attend a conference from Chiesi and their employer has received payment for lecturing from Chiesi. K.C. and K.C. have no financial or conflicts to declare.
- 1Delivering a ‘Net Zero’ NHS. NHS England. 2020.https://pharmaceutical-journal.com/article/opinion/how-switching-a-handful-of-patients-inhalers-saved-nearly-5000kgco2e (accessed Aug 2022).
- 2The NHS Long Term Plan. NHS. 2019.https://www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf (accessed Aug 2022).
- 3Network Contract Directed Enhanced Service — Investment and Impact Fund 2022/23: Updated Guidance. NHS England. 2022.https://www.england.nhs.uk/wp-content/uploads/2022/03/B1357-investment-and-impact-fund-2022-23-updated-guidance-march-2022.pdf (accessed Aug 2022).
- 4Pritchard J, Usmani O. The Greenest Inhaler: A Patient-centric Approach. EMJ Respiratory. 2022.https://emj.emg-health.com/wp-content/uploads/sites/2/2022/05/The-Greenest-Inhaler-A-Patient-centric-Approach-1.pdf (accessed Aug 2022).
- 5Jeswani HK, Azapagic A. Life cycle environmental impacts of inhalers. Journal of Cleaner Production. 2019;237:117733. doi:10.1016/j.jclepro.2019.117733
- 6Prescription Cost Analysis – England 2020/21. NHS Business Services Authority. 2021.https://www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis-england/prescription-cost-analysis-england-202021 (accessed Aug 2022).
- 7Wilkinson AJK, Braggins R, Steinbach I, et al. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open. 2019;9:e028763. doi:10.1136/bmjopen-2018-028763
- 8Kluger J. How One Commonly Used Asthma Inhaler is Damaging the Planet. Time. 2019.https://time.com/5717676/asthma-inhalers-and-climate/ (accessed Aug 2022).
- 9Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: So much for a global policy. Respiratory Medicine. 2011;105:1099–103. doi:10.1016/j.rmed.2011.03.012
- 10Koura opens world’s first HFA 152a medical propellant production facility. Chiesi. 2022.https://www.chiesi.com/en/koura-opens-worldrs-first-hfa-152a-medical-propellant-production-facility/ (accessed Aug 2022).
- 11Barnes PJ. Inhaled Corticosteroids. Pharmaceuticals. 2010;3:514–40. doi:10.3390/ph3030514
- 12Bloom CI, Cabrera C, Arnetorp S, et al. Asthma-Related Health Outcomes Associated with Short-Acting β2-Agonist Inhaler Use: An Observational UK Study as Part of the SABINA Global Program. Adv Ther. 2020;37:4190–208. doi:10.1007/s12325-020-01444-5
- 13Greener inhalers: are we setting the wrong targets? Pharmaceutical Journal. 2021. doi:10.1211/pj.2021.1.110294
- 14Wilkinson A, Woodcock A. The environmental impact of inhalers for asthma: A green challenge and a golden opportunity. Brit J Clinical Pharma. 2021;88:3016–22. doi:10.1111/bcp.15135
- 15Engelkes M, Janssens HM, de Jongste JC, et al. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2014;45:396–407. doi:10.1183/09031936.00075614
- 16Price D, Chrystyn H, Kaplan A, et al. Effectiveness of Same Versus Mixed Asthma Inhaler Devices: A Retrospective Observational Study in Primary Care. Allergy Asthma Immunol Res. 2012;4:184. doi:10.4168/aair.2012.4.4.184
- 17Bosnic-Anticevich S, Chrystyn H, Costello R, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. COPD. 2016;Volume 12:59–71. doi:10.2147/copd.s117196
- 18Basheti IA, Armour CL, Bosnic-Anticevich SZ, et al. Evaluation of a novel educational strategy, including inhaler-based reminder labels, to improve asthma inhaler technique. Patient Education and Counseling. 2008;72:26–33. doi:10.1016/j.pec.2008.01.014
- 19Capstick TGD, Azeez NF, Deakin G, et al. Ward based inhaler technique service reduces exacerbations of asthma and COPD. Respiratory Medicine. 2021;187:106583. doi:10.1016/j.rmed.2021.106583
- 20Usmani O, Capstick T, Saleem A. Choosing an appropriate inhaler device for the treatment of adults with asthma or COPD. MGP guidelines. 2020.https://www.guidelines.co.uk/respiratory/inhaler-choice-guideline/455503.article (accessed Aug 2022).
- 21Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246–51. doi:10.1183/09031936.02.00218402
- 22Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respiratory Medicine. 2011;105:930–8. doi:10.1016/j.rmed.2011.01.005
- 23Lavorini F, Magnan A, Christophe Dubus J, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respiratory Medicine. 2008;102:593–604. doi:10.1016/j.rmed.2007.11.003
- 24Liberating the NHS: No decision about me, without me. Department of Health. 2012.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216980/Liberating-the-NHS-No-decision-about-me-without-me-Government-response.pdf (accessed Aug 2022).
- 25Thomas M, Price D, Chrystyn H, et al. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009;9. doi:10.1186/1471-2466-9-1
- 26Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Review of Respiratory Medicine. 2012;6:91–103. doi:10.1586/ers.11.89
- 27Al-Showair RAM, Tarsin WY, Assi KH, et al. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respiratory Medicine. 2007;101:2395–401. doi:10.1016/j.rmed.2007.06.008
- 28Al-Showair RAM, Pearson SB, Chrystyn H. The Potential of a 2Tone Trainer To Help Patients Use Their Metered-Dose Inhalers. Chest. 2007;131:1776–82. doi:10.1378/chest.06-2765
- 29Newman S, Woodman G, Clarke S, et al. Effect of InspirEase on the deposition of metered-dose aerosols in the human respiratory tract. Chest 1986;89:551–6. doi:10.1378/chest.89.4.551
- 30CHRYSTYN H. Is inhalation rate important for a dry powder inhaler? using the In-Check Dial to identify these rates. Respiratory Medicine. 2003;97:181–7. doi:10.1053/rmed.2003.1351
- 31British guideline on the management of asthma. Clinical guideline 158. British Thoracic Society/Scottish Intercollegiate Guidelines Network. 2019.https://www.sign.ac.uk/our-guidelines/british-guideline-on-the-management-of-asthma/ (accessed Aug 2022).
- 32Chronic obstructive pulmonary disease in over 16s: diagnosis and management, NICE guideline [NG115]. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/NG115 (accessed Aug 2022).
- 33How to Reduce the Carbon Footprint of Inhaler Prescribing: A Guide for Healthcare Professionals in the UK. Asthma UK/British Heart Foundation. 2021.https://s40639.pcdn.co/wp-content/uploads/Reducing-Carbon-Footprint-of-Inhaler-Prescribing-v3.3.2.pdf (accessed Aug 2022).
- 34Melani AS, Zanchetta D, Barbato N, et al. Inhalation technique and variables associated with misuse of conventional metered-dose inhalers and newer dry powder inhalers in experienced adults. Annals of Allergy, Asthma & Immunology. 2004;93:439–46. doi:10.1016/s1081-1206(10)61410-x
- 35Basheti IA, Salhi YB, Basheti MM, et al. <p>Role of the pharmacist in improving inhaler technique and asthma management in rural areas in Jordan</p> CPAA. 2019;Volume 11:103–16. doi:10.2147/cpaa.s213271
- 36Basheti I, Reddel H, Armour C, et al. Counseling about turbuhaler technique: needs assessment and effective strategies for community pharmacists. Respir Care 2005;50:617–23.https://www.ncbi.nlm.nih.gov/pubmed/15871755
- 37Chiesi launches postal asthma inhaler recycling scheme. Pharmaceutical Journal. 2021. doi:10.1211/pj.2021.20208795
- 38Ovchinikova L, Smith L, Bosnic-Anticevich S. Inhaler Technique Maintenance: Gaining an Understanding from the Patient’s Perspective. Journal of Asthma. 2011;48:616–24. doi:10.3109/02770903.2011.580032