Shutterstock
After reading this article, you should be able to:
- Understand a patient’s journey from foetal to paediatric life;
- Describe how medicines are used to manage congenital heart disease;
- Understand how the pharmacy team contributes to patient care;
- Consider the parent and patient voice throughout the patient’s treatment.
This case study describes the journey of a patient with congenital heart disease (CHD) from foetal to paediatric care and gives the perspective of the parents and the medical team involved in the patient’s care. The medicines that are used to manage CHD are summarised and the involvement from the pharmacy team are highlighted. The transitional care from paediatric into adult care is not discussed in this article. For information on this see ‘Congenital heart disease standards and specifications’ and the Health Education England’s CHD e-learning programme1,2.
This is the second article of a series on CHD. The previous article ‘Congenital heart disease: an overview’, described how CHD is defined; highlighted its prevalence; demonstrated how the heart is formed in utero; and outlined some of the causes and diagnostic tests used. It provided an overview of the common types of CHD and some of the medications used to treat the condition.
Each cardiac lesion can vary in its severity and presentation from patient to patient, therefore the surgical or medical approach may differ between patients. The medical and surgical treatments offered are discussed between a multidisciplinary team of experts. There is a robust governance system in place to maintain high-quality patient care, but there may be different approaches between tertiary cardiac surgical centres.
Initial case presentation
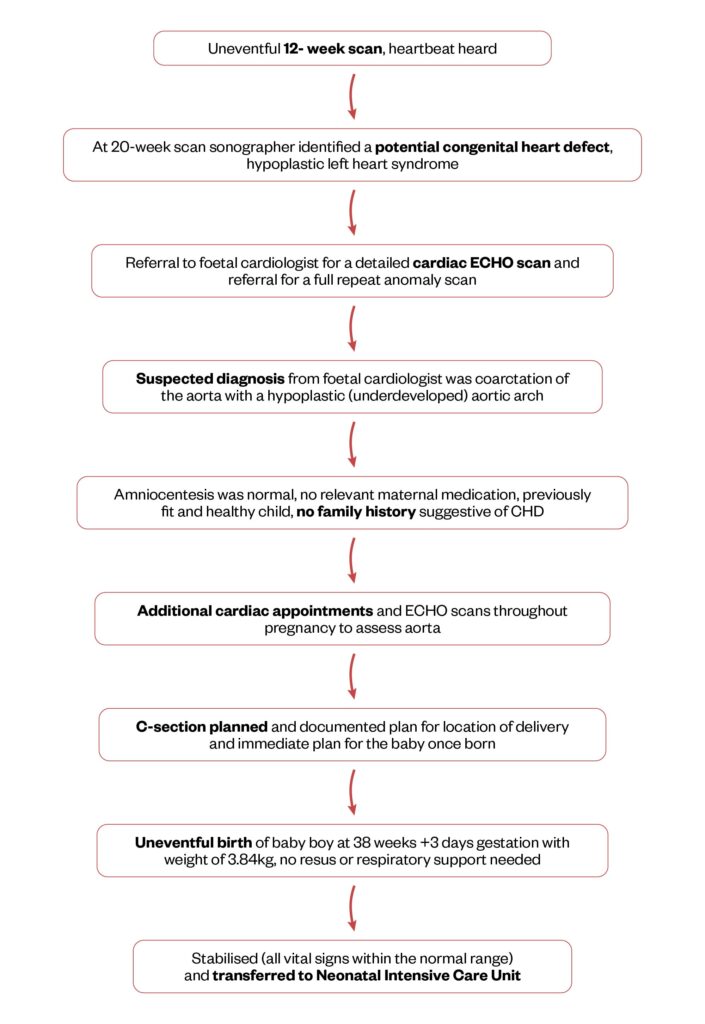
In utero, ‘Baby I’ was identified at the 20-week scan as having a potential cardiac anomaly3,4. The sonographer initially suspected hypoplastic left heart syndrome, a condition where the left ventricle is underdeveloped and is smaller than usual, meaning that it cannot support adequate systemic circulation5.
“At the 20-week scan, I had an inkling that something was up, I could see from the screen that the sonographer had been scanning the heart for a while. I knew something was wrong. I was told that a member of the cardiac team would be in contact. The sonographer told me not to look in my medical notes. Of course, I looked and it said ‘Hypoplastic left heart’. I felt lost and didn’t know what to do and wanted answers.”
This necessitated a referral to cardiology specialists, with whom a detailed plan was developed for Baby I’s delivery, which included immediate admission to a neonatal intensive care unit (NICU).
Figure 1 shows each stage in Baby I’s journey from diagnosis to birth and admission to NICU3–7.
Figure 1: Baby I’s journey from foetal life with investigations to his birth
“During pregnancy all the scans were really draining, I had a scan every four weeks or so, I was absolutely exhausted after the scans because of the emotions. I cried so much after every scan.
“My main concerns when I was pregnant was how it was going to affect my daughter who was two years old and about concerns of being in hospital without her.”
Initial management after the birth
Following delivery, Baby I was transferred to the NICU for two days (day zero being the day of birth).
Baby I was transferred to the NICU to complete a full body assessment, start critical medical treatment with intensive monitoring, and confirm the antenatally diagnosed cardiac anomaly. The initial assessments carried out can be seen in Box 1.
Box 1: Initial assessments
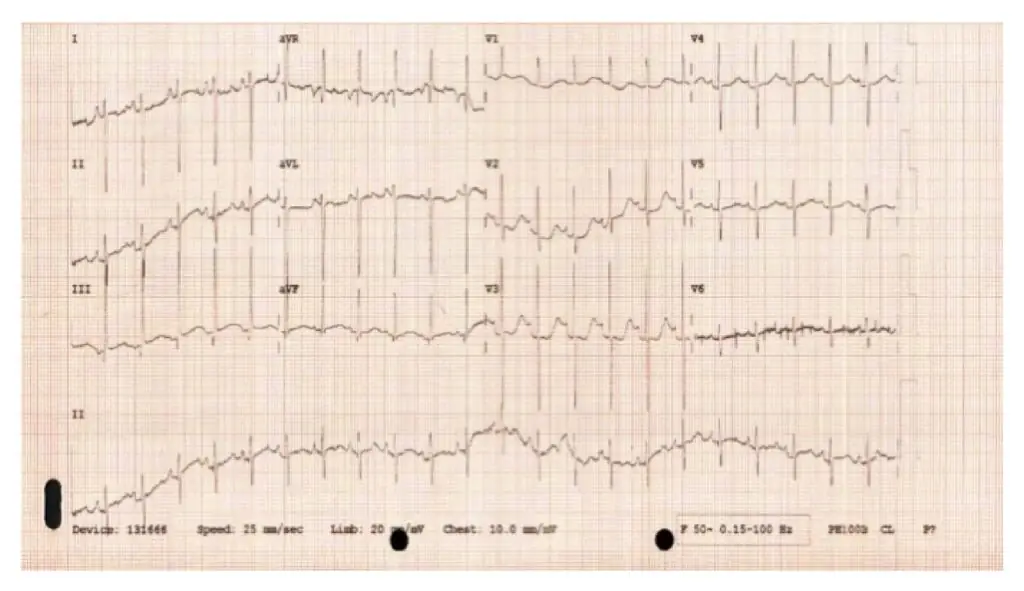
- Cardiac echocardiography showed a hypoplastic aortic arch and coarctation of the aorta (see Figure 2);
- No murmur heard;
- Femoral pulses felt (patent ductus arteriosus);
- Lactate stable (high levels can indicate shock or heart failure);
- Blood tests showed normal renal, haematological and electrolyte levels;
- Cardiovascular vitals signs, such as blood pressure and heart rate, were within normal levels;
- Temperature 36.7 degrees, showing no signs of infection;
- No signs of respiratory distress (no respiratory support required).
Figure 2: Baby I’s ECG showing a sinus rhythm, showing no concerns and normal conduction of the electrical activity within the heart
“Everyone made me feel safe in theatre, the anaesthetist kept me calm. My husband and I got to have a cuddle before they took our son to NICU. NICU nurses were amazing and allowed me to cuddle him and they looked after me too.”
An umbilical venous catheter was inserted so that critical medications could be administered. This is where the IV line is placed into the umbilical vein via the umbilical stump. It gives central IV access for medication and fluids for up to the first ten days of neonatal life8. There were concerns about poor blood flow (perfusion) to the stomach, so Baby I was kept nil by mouth and an IV glucose infusion was started owing to potential risk of necrotising enterocolitis (NEC)9. More information on NEC can be found in ‘Gastrointestinal complications associated with prematurity’.
A continuous IV dinoprostone infusion was started to keep the patent ductus arteriosus (PDA) open, facilitating improved systemic blood flow until surgical correction10–12. Keeping the ductus arteriosus open is advantageous (and can be life-saving) because it facilitates improved systemic blood flow until surgical correction of the coarctation of the aorta can occur13. A summary of Baby I’s medications can be seen in Box 2.
Box 2: Medications prescribed on NICU (Baby I birth weight 3.84kg)
- Dinoprostone: 3 nanograms/kg/minute. Using 100 micrograms in 50mL solution. Run continuous IV infusion via a syringe driver pump at 0.35mL/hr, administered via umbilical catheter;
- Glucose 5%: Run continuous IV infusion via a syringe driver pump at 0.65mL/hr to make up to 1mL/hr (with the dinoprostone) to maintain line patency, administered via umbilical catheter;
- Glucose 10%: 60ml/kg/day. Run continuous IV infusion via an IV bag at 9.6 ml/hr, administered via umbilical catheter.
Baby I’s baseline vital signs were monitored in addition to ECG monitoring, respiration rate, oxygen saturations, temperature, blood gases and blood sugars. The umbilical venous catheter used to administer dinoprostone and intravenous glucose infusion was monitored for signs of redness/infection and patency. The syringe pump flow rate readings were reviewed as per usual medicines policy to ensure correct delivery of the critical drug dinoprostone.
Preparation for surgery
“I had a great team of nurses, pharmacists and doctors who took care of my baby, I felt that my baby was in safe hands and cared for. I found it difficult being there on day three when my milk came in and very emotional trying not to cry, when you are emotionally worried about your baby and also all the hormones that come with just giving birth.”
On day two, Baby I was transferred to the specialist tertiary surgical centre high-dependency cardiac ward, at the children’s hospital for further management. Baby I underwent further investigations — including an ECG (see Figure 2), chest x-ray (see Figure 3) and repeat echocardiography (see Figure 4)12 — to gather data on their condition to present to the joint cardiac conference, a multidisciplinary team meeting tasked with deciding the most suitable surgical or medical treatment option, and the urgency of the intervention.
Figure 3: Baby I’s chest x-ray showing mild heart enlargement, and under inflated, slightly ‘wet’ lungs. A nasogastric tube and umbilical line are present
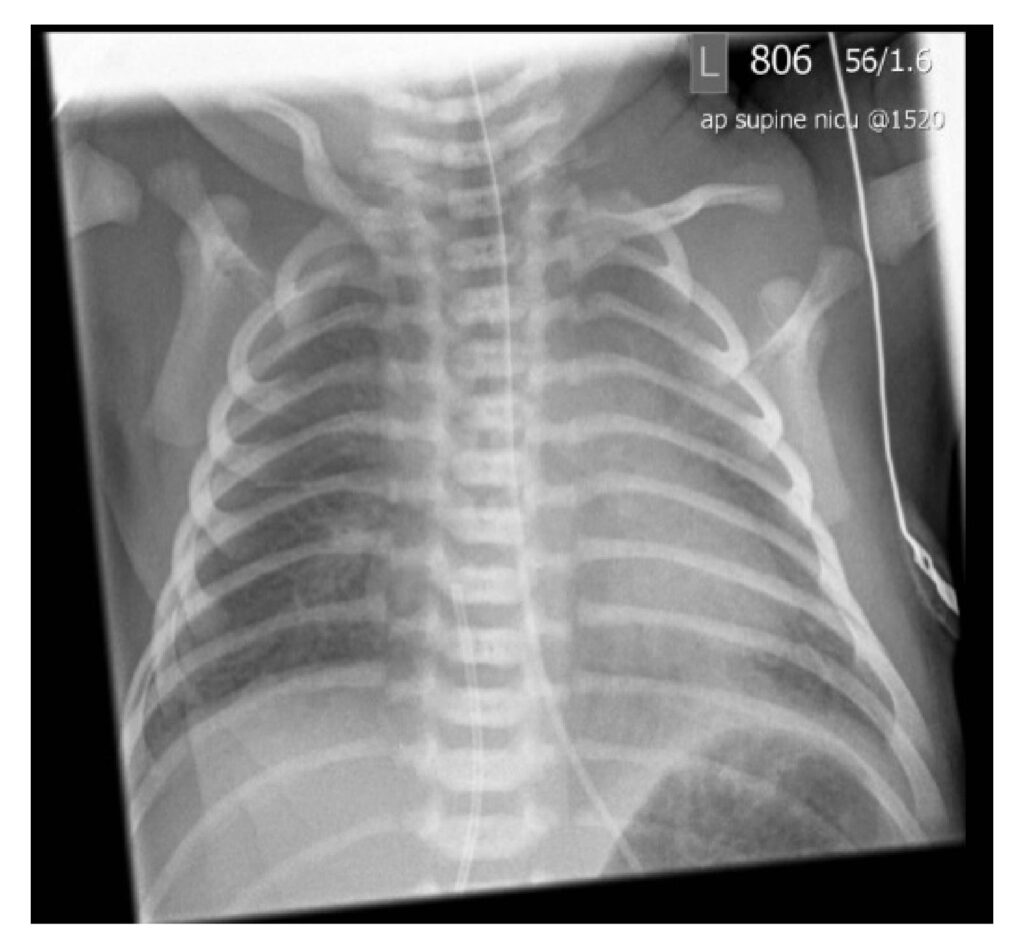
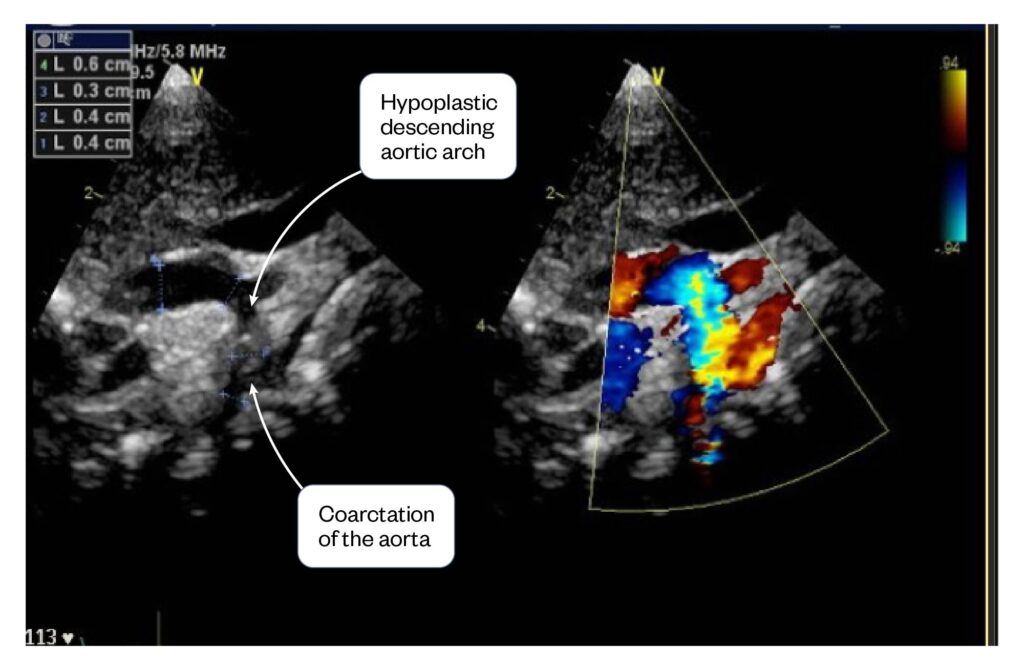
Figure 4: Baby I’s echocardiography showing the descending hypoplastic aortic arch and the coarctation of the aorta (left image) and the right image is showing the flow of blood, the yellow colour indicates the turbulent flow of blood through the narrowed section of aorta
“The consultant cardiologist I had was really good from my maternity appointments to coming to the cardiac high dependency ward, explained everything to me, always gave me options, explained what might be expected at the next scan, I felt safe with her.”
It was decided that Baby I should receive corrective surgery to enlarge the aorta using a patch. He was stable without concern, so emergency surgery was not required, but planned for day nine and to remain on a dinoprostone infusion, until surgical correction had taken place.
“I remember waiting for the surgery and after each scan hoping they would say he wouldn’t have to undergo open heart surgery.”
On day three, Baby I developed a slight rise in temperature to 37.8°C. A temperature increase could be a side effect of dinoprostone10,11; however, owing to having additional risks, such as an IV line in situ and pending cardiac surgery, the decision was made to take blood cultures and start intravenous gentamicin and benzylpenicillin14–16. Care was needed to avoid anti-pyretic treatment, because both paracetamol and ibuprofen can close a PDA, which needed to be kept patent17.
Gentamicin was started on day three and a trough level was taken prior to the second dose which showed normal levels. The antibiotics were stopped on day six, as blood cultures were negative showing no growth of organisms and Baby I had no further spikes in temperature or other indications of raised inflammatory markers (e.g. C-reactive protein)18.
On day three, owing to ongoing concerns about poor perfusion of the stomach and the risk of necrotising enterocolitis, the multidisciplinary team decided to commence parenteral nutrition (PN)9. PN is an IV solution containing nitrogen, carbohydrate, lipid, electrolytes, vitamins and trace elements19. PN blood tests were taken prior to starting PN and daily for the first few days of treatment, with weight and fluid balance being monitored daily. PN and dinoprostone were continued until surgical repair.
Diuretics were started after a chest x-ray showed a ‘wet lung’ and some signs of increased respiration rate. These were continued until surgical repair, with close monitoring of renal function, electrolytes, fluid balance and Baby I’s weight. The dinoprostone dose was increased on day five, based on echocardiography showing the ductus arteriosus beginning to close and lactate levels increasing. The continuous glucose 5% flush was stopped as sufficient volume was flowing through the catheter (>1mL/hr dinoprostone). Monitoring of ECG, respiration rate, oxygen saturations, temperature, blood gases and blood sugars continued. A summary of the medications used while Baby I was in the cardiac high dependency unit can be seen in Box 3.
On day five, Baby I had his newborn blood spot, which screens for serious conditions such as cystic fibrosis, the results of which all came back negative20.
Box 3: Medications prescribed on cardiac high-dependency unit (using Baby I birth weight 3.84kg)
- Dinoprostone 10 nanograms/kg/minute. Using 100 micrograms in 50mL glucose 5%. Run continuous IV infusion via a syringe driver pump at 1.2 mL/hr, administered via umbilical catheter;
- Gentamicin 5mg/kg 36 hourly. 19mg 36 hourly, administered via peripheral cannula;
- Benzylpenicillin 50mg/kg 12-hourly. 190mg 12-hourly, administered via a peripheral cannula;
- Parenteral nutrition 100mL/kg/day. 16mL/hr, administered via umbilical catheter;
- Furosemide (50mg/5mL) 1mg/kg/dose. 4mg 12-hourly enterally;
- Spironolactone (50mg/5mL) 1mg/kg/dose. 4mg 12-hourly enterally.
“After discussion with the surgeon it all felt very real about what was going to happen, and we were very anxious. I remember saying to my mum that I don’t want him to die, as I love him so much already. The day of surgery was the hardest, because the worry was then real. My husband and I were provided with good explanations of what was going to happen to him in surgery, but understanding all of this was also very scary.”
Surgery took place on day nine to repair Baby I’s coarctation of the aorta and hypoplastic aortic arch.
The surgical procedure took place via median sternotomy (excision down the centre of the chest through the sternum) and involved cardiopulmonary bypass (diversion of blood around the heart and lungs)21. The aorta was resected, widened using a patch to repair the narrowing of the hypoplastic aortic arch and coarctation of the aorta and the patent ductus arteriosus was ligated (tied off).
Mediastinal and pericardial drains were inserted to prevent fluid build-up around the heart, and temporary pacing wires were inserted with leads to the atria and ventricles, to manage cardiac rhythm in the postoperative period. The surgery was successful, and Baby I remained in sinus rhythm throughout. Dinoprostone and PN were stopped. Baby I was transferred from theatre to the paediatric intensive care unit (PICU) for postoperative care (see Figure 5).
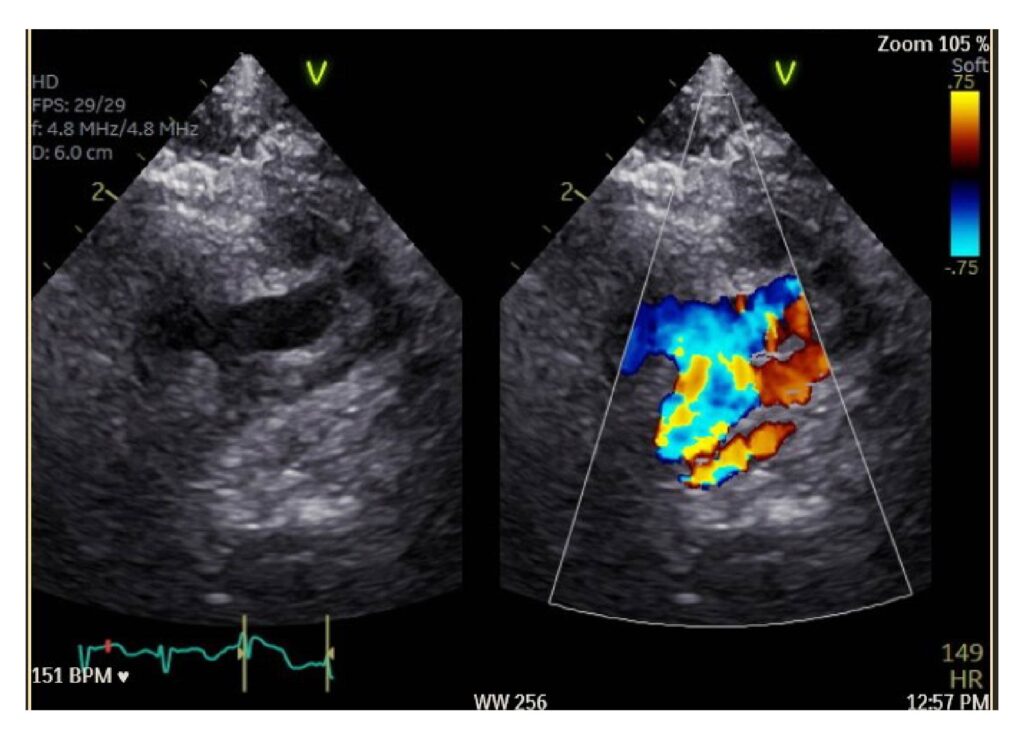
Figure 5: Baby I’s post operative echocardiography showing the repair of the hypoplastic arch and coarctation (left image) and the right image shows less turbulent flow of blood (less yellow colour)
Postoperative management
Baby I remained on PICU for three days to recover from the surgical period. He received medical treatment to support heart function and intensive monitoring was provided.
He returned from theatre intubated and ventilated. Flucloxacillin was administered to prevent Staphlococcus aureus infections, postoperatively as microbial cover, to prevent surgical wound infections22.
Morphine and midazolam were used to provide pain relief and sedation, these were discontinued after two days, so weaning was not required.
“During the surgery was just a nervous wait, we were lucky enough to live close to the hospital therefore just went home, surgery was around five hours long. Again, we were very lucky to have a caring nurse who explained everything to us, then the surgeon came and said the surgery went well and he was a strong boy.”
Milrinone and dopamine were used to support cardiac function. These have different functions: milrinone is a positive inotrope and vasodilator, a phosphodiesterase type 3 inhibitor in cardiac and smooth muscle, promoting cardiac contractility23. Dopamine is a positive inotrope exerting its action on the heart via beta-adrenergic receptors, increasing cardiac contractility24. These were stopped after two days. Unfractionated heparin was started as a prophylactic infusion owing to a patch being placed to repair the aorta, as Baby I was not on sufficient volumes of enteral feeding, aspirin could not be started, which was the cardiac surgeon’s initial plan. A summary of the medications prescribed in the PICU post-surgery can be seen in Box 425.
Box 4: Medications prescribed on paediatric intensive care unit post-surgical repair (using Baby I birth weight 3.84kg)
- Flucloxacillin (prophylactic) 25mg/kg/dose. 100mg 8-hourly IV for a total of 24 hours;
- Morphine 20 micrograms/kg/hour. 3.9mg in 50mL of sodium chloride 0.9%. Continuous 1mL/hr;
- Midazolam 25 microgram/kg/hour. 19.5mg in 50ml of glucose 5%. Continuous 0.25mL/hr;
- Milrinone 0.25micrograms/kg/minute.5.9mg in 50ml glucose 5%. Continuous 0.5mL/hr;
- Dopamine 5 micrograms/kg/minute. 59mg in 50ml of glucose 5%. Continuous 1mL/hr;
- Heparin (prophylactic) 10 units/kg/hr. 3,900 units in 50mL sodium chloride 0.9%. Continuous 0.5mL/hr;
- Furosemide (50mg/5mL) 1mg/kg/dose. 4mg 12-hourly enterally;
- Spironolactone (50mg/5mL) 1mg/kg/dose. 4mg 12-hourly enterally;
- Paracetamol 15mg/kg/dose. 60mg 8-hourly prn enterally;
- Plasmalyte 148 + glucose 10% IV fluids were titrated to fluid allowance with infusions/flush volumes above and with enteral feeds (expressed breast milk) when they were cautiously introduced on day ten.
Baby I continued to have his vital signs continuously monitored in addition to regular echocardiography to assess the repair, heart function and when chest drains, and pacing wires were removed. Blood tests showed mild renal impairment with an increased urea and creatinine that resolved with careful fluid management.
After successfully extubating on day 13, Baby I was transferred to the cardiac high dependency unit (HDU), this was because he no longer had care needs required on PICU, such as no longer being ventilated or having chest drains in situ.
“Being on the ward with other families, everyone does speak to each other and has care for one another and compassion about what you are going through, as they are probably the only people that truly understand what you are feeling and the worries that you have.”
On day 13, Baby I had heparin discontinued when on sufficient enteral feeds to tolerate aspirin, at a dose of 5mg/kg/day, rounded to 20mg 24-hourly for three months, as per the cardiac surgeon’s plan26.
Routine blood tests were conducted to review renal function and electrolytes to assess if the renal impairment had resolved. The multidisciplinary high-dependency team reviewed all the investigations, vital signs, medication and stepped down Baby I to a ward cardiac bed on day 14. This was possible because he no longer triggered criteria for an HDU bed, he was progressing well post-cardiac repair and there were no clinical concerns.
Preparation for discharge
On the general ward, Baby I continued to improve and establish full enteral feeding.
The focus was to prepare him for discharge home, which involved repeating tests and investigations to monitor his progress, stabilising and optimising his medicines, where necessary and supporting his parents to manage his care independently. The necessary pre-discharge checks can be seen in Box 5.
Box 5: Ongoing monitoring/pre-discharge checks
- Cardiac echocardiography showed a good repair and good cardiac function;
- ECG-showed sinus rhythm;
- Chest x-ray showed no indication of fluid or infection;
- Blood tests showed normal renal, haematological and electrolyte levels;
- Cardiovascular vital signs, such as blood pressure and heart rate, were within normal levels;
- Temperature normal;
- Surgical wound site looked clean;
- Feeding well and starting to have expected weight gain.
A supply of the following medicines were prepared, and the parents were counselled to safely prepare and administer each medicine. These can be seen in Box 6.
Box 6: Medications prescribed for discharge (based on Baby I’s weight at time of discharge — 3.9kg)
- Furosemide (50mg/5mL liquid) 1mg/kg/dose. 4mg 12-hourly enterally, using an oral syringe;
- Spironolactone (50mg/5mL liquid) 1mg/kg/dose. 4mg 12-hourly enterally, using an oral syringe;
- Aspirin (using 75mg dispersible tablets). 5mg/kg/dose. 20mg 24-hourly enterally for 3 months. Disperse a 75mg tablet in 3mL of water and give 0.8mL via an oral syringe, discarding the remaining solution.
The discharge summary was completed with details of the surgical repair, relevant investigations and discharge medications and copies sent to Baby I’s GP and other healthcare professionals involved in their care. A copy was also given to the parents. Medication was ordered and counselling for each medication with relevant supporting information and any relevant communication with other healthcare professionals (e.g. GP sternotomy wound review and appropriate weight gain). Baby I was discharged home on day 15, with a two-week follow-up in clinic, with his paediatric cardiologist and safety netting advice if any parental concerns.
“I felt truly lucky being in a place where I had a lot of friends and visitors and was looked after and trusted everyone. I do feel that for other people they might not have that. Grateful that visiting hours were any time and that there was a playroom for siblings.”
Longer term follow-up
Baby I was seen regularly in clinic, initially two weeks post-discharge, then every three to six months depending on the cardiologist’s investigations. The diuretics furosemide and spironolactone were stopped after the first clinic appointment and the aspirin was continued for three months at the request of the cardiac surgeon, owing to patch placement.
Baby I had regular clinic appointments with echocardiography, there was some concern about narrowing of the aorta and some left ventricular hypertrophy (thickening of the left ventricle wall). Aged five months, he had a balloon dilation of the re-coarctation via a cardiac catheterisation procedure, which was successful to try and correct the narrowed portion of the aorta27.
Subsequent follow-ups have been annual, with general safety-netting advice of good dental care; early treatment of any skin infections; to avoid any body piercings or tattoos (once relevant), owing to the risk of endocarditis28,29. The parents were advised to encourage Baby I to maintain a good level of exercise and to maintain his cardiovascular health. Baby (now child) I’s systolic blood pressure was monitored on several clinic visits and found to be high. Owing to this hypertension, a low dose of amlodipine was started at 2.5mg 24-hourly30. This continued to be reviewed annually at each clinic appointment, deciding whether optimisation and titration would be required.
Pharmacy involvement
There was a wide range of pharmacy staff involved in the care of the patient and family of Baby I and his CHD journey. This included assistant technical officers to technicians on the ward, in the dispensary, aseptic department, purchasing, stock management, governance team, in hospital, GP and community services. Baby I’s case demonstrates the range of pharmacists who have impacted his care; see the Table below for an outline of their roles.
Table 1: The different roles of pharmacists involved in Baby I’s care
The various pharmacists mentioned in the Table — in particular the clinical neonatal, paediatric cardiac and paediatric intensive care pharmacists — had an active role in the multidisciplinary team for Baby I’s pharmaceutical care needs, as the medication expert, with the nursing and medical staff.
Considerations for neonatal and paediatric patients
This case demonstrates that there are several additional considerations when treating neonatal and paediatric patients medically. These include administration of IV infusions, compatibility and line type, formulation choice, supporting patients and their families for discharge and transition to primary care.
IV infusions
Whilst Baby I was on NICU, PICU and the cardiac HDU unit, several IV medications were given, such as dinoprostone, benzylpenicillin, gentamicin, flucloxacillin, milrinone, morphine, midazolam and unfractionated heparin. A useful resource used to aid nursing staff for IV paediatric administration is Medusa, which provides information on IV compatibility. It is important to also consider the line type, whether it is a peripheral or central line and whether it needs a continuous infusion to keep the line patent31.
Formulation
During Baby I’s time as an inpatient and on discharge, off-label or unlicensed formulations were used, manipulation of adult dosage forms required, excipient content formulation assessment and use of standardised liquid concentration was needed32–34.
Baby I was discharged home with an unlicensed spironolactone liquid. The use of unlicensed medicines or licensed medicines outside of the product licence (off label) is necessary and common in paediatric practice, when there is no suitable alternative. Paediatric patients are therefore using adult formulations32,33.
For Baby I, Medicines for Children (MFC) leaflets were supplied for furosemide, spironolactone and aspirin35. Aspirin is contra-indicated in children aged under 16 years owing to an increased risk of Reye’s syndrome and the MFC leaflet explains this in easy-to-understand language for the parents and carers36,37.
Additional information on the use of unlicensed medicines or licensed medicines for unlicensed applications in paediatric practice can be found in the Royal College of Paediatrics and Child Health (RCPH)’s ‘The use of unlicensed medicines or licensed medicines for unlicensed applications in paediatric practice’32.
Baby I was using a preservative free unfractionated heparin formulation and was discharged home with a furosemide liquid containing ethanol that had been assessed for suitability. Consideration needs to be given to the excipients in oral liquids. Excipients have different roles; for example, enhancing stability, bioavailability, product identification (colouring), taste or as a preservative. These excipients can potentially cause harm, so the oral liquid needs to be assessed for suitability for the neonatal or paediatric patient. See RCPH/Neonatal and Paediatric Pharmacists Group (NPPG) position statement for more information33. The medicines given to Baby I’s parents on discharge had already been assessed for suitability for neonatal and paediatric use, Baby I’s parents were counselled about getting different oral liquids from their GP and how the concentration and excipient profile could be different.
Baby I was discharged home with furosemide and spironolactone 50mg/5mL oral liquids. The parents were counselled on different strengths, the discharge summary clearly documented the strength supplied, and the local formulary website documents strengths used in paediatric cardiology. There are several different concentrations of liquids e.g. furosemide liquid 20mg/5mL, 40mg/5mL and 50mg/5mL, leading to risks in under and overdosing. The hospital may discharge on a certain concentration, but a GP surgery may not have that strength on their prescribing system, leading to confusion to the parents and potential risk to the patient. See RCPH/NPPG position statement for more information33.
Another important consideration is the ability to be able to swallow tablets or capsules. As the patient grows and develops and continues their congenital heart disease journey, the patient may be started on new medications; for example, in Baby I’s case, amlodipine to control blood pressure. Teaching children to be able to swallow tablets provides many benefits; a skill developed for life, reduce the need for unlicensed liquid and problematic excipients or manipulation of adult formulations, such as crushing tablets, and has a potential cost saving, as well as reducing fridge storage issues, short liquid expiries and better availability in local pharmacies. There is also less environmental impact of tablet production compared to liquids38. Kidzmed is an initiative being used to promote the teaching of swallowing tablets39.
Transition to primary care
Good communication with clear discharge summaries, including detail on investigations and monitoring required, medication formulations documented, doses and review dates or duration are essential for seamless patient care. Shared formularies with additional guidance as to who is providing care and what to do if there are any complications of treatment or parenteral concern40,41.
Parents and carers will receive paper or electronic copies of discharge summaries and clinic appointments, educational material if required, these are shared with their local paediatricians, GPs and any other relevant healthcare professionals. There will be contacts given to parents and carers of who to contact if any concerns between clinic appointments. Electronic records of clinic appointments and investigations can be shared electronically between lead cardiac centres, district generals and relevant healthcare professionals.
Summary
The wide breadth of pharmacy involvement in Baby I’s CHD journey from foetal to paediatric life highlights the huge impact the pharmacy team have in CHD and their roles in developing quality care, a better patient experience, putting the patient at the centre of what we do.
Listening to the parent or patient voice is vital to better understand how we can advance the services we offer. A strong message that came through from the parent of Baby I is how supported she was, how compassionate the healthcare team were and how communication was essential to explaining next steps.
Useful resources
Medicines for Children, which is run in partnership with the Royal College of Paediatrics and Child Health, Neonatal and Paediatric Pharmacists Group and WellChild, is aimed to give parents and carers needed better access to information on children’s medicines. Its leaflets and general medication information is available on a website, an app and is linked in the BNF for Children.
The NPPG aims to provide more quality care of patients by developing pharmacists with education and sharing practice and has a newly started paediatric cardiology group, where pharmacists who specialise in this area or have an interest in paediatric cardiology share expertise and drive the national agenda.
Acknowledgements
First, the author would like to give a heartfelt thanks to the parents of Baby I, who consented for their son’s congenital heart disease journey to be shared, including medical images and sharing their feelings during a very challenging time.
Second, thank you to Teresa Brooks and Nicola Husain for their support for this article.
- 1.Congenital heart disease standards and specifications. NHS England. 2016. Accessed May 2025. https://www.england.nhs.uk/wp-content/uploads/2018/08/Congenital-heart-disease-standards-and-specifications.pdf
- 2.NHS England Learning Hub. NHS England. Accessed May 2025. https://learninghub.nhs.uk/
- 3.Screening tests in pregnancy . NHS. 2021. Accessed May 2025. https://www.nhs.uk/pregnancy/your-pregnancy-care/screening-tests/
- 4.20-week screening scan. NHS England. 2024. Accessed May 2025. https://www.gov.uk/government/publications/fetal-anomaly-screening-programme-handbook/20-week-screening-scan
- 5.Heart Conditions in Children – Hypoplastic Left Heart Syndrome (HLHS) Information Sheet. Children’s Heart Federation. Accessed May 2025. https://chfed.org.uk/heart-conditions-in-children-hypoplastic-left-heart-syndrome-hlhs-information-sheet/
- 6.Coarctation of the Aorta (CoA). American Heart Association. Accessed May 2025. https://www.heart.org/en/health-topics/congenital-heart-defects/about-congenital-heart-defects/coarctation-of-the-aorta-coa
- 7.Zierler S. Maternal drugs and congenital heart disease. Obstet Gynecol. 1985;65(2):155-165. https://www.ncbi.nlm.nih.gov/pubmed/3881709
- 8.Umbilical catheters. NHSGGC Paediatrics for Health Professionals. 2023. Accessed May 2025. https://clinicalguidelines.scot.nhs.uk/ggc-paediatric-guidelines/ggc-paediatric-guidelines/neonatology/umbilical-catheters/
- 9.Lin PW, Stoll BJ. Necrotising enterocolitis. The Lancet. 2006;368(9543):1271-1283. doi:10.1016/s0140-6736(06)69525-1
- 10.Dinoprostone. BNF for Children. Accessed May 2025. https://bnfc.nice.org.uk/drugs/dinoprostone/
- 11.Prostin E2 Sterile Solution 1mg/mL IV. Electronic Medicines Compendium. 2024. Accessed May 2025. https://www.medicines.org.uk/emc/product/1659/smpc
- 12.Congenital heart disease. NHS. 2021. Accessed May 2025. https://www.nhs.uk/conditions/congenital-heart-disease/
- 13.Patent ductus arteriosus. BMJ Best Practice. 2025. Accessed May 2025. https://bestpractice.bmj.com/topics/en-gb/766
- 14.Neonatal infection: antibiotics for prevention and treatment. National Institute for Health and Care Excellence. 2024. Accessed May 2025. https://www.nice.org.uk/guidance/ng195
- 15.Benzylpenicillin sodium. BNF for Children. Accessed May 2025. https://bnfc.nice.org.uk/drugs/benzylpenicillin-sodium/
- 16.Gentamicin. BNF for Children. Accessed May 2025. https://bnfc.nice.org.uk/drugs/gentamicin/
- 17.Guideline for the management of Patent Ductus Arteriosus. North West Neonatal Operational Delivery Network. 2020. Accessed May 2025. https://www.neonatalnetwork.co.uk/nwnodn/wp-content/uploads/2020/10/GL-ODN-09-NW-Guideline-for-the-Management-of-PDA.pdf
- 18.Singh B, Goyal A, Patel B. statpearls. Published online May 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK441843/
- 19.Neonatal parenteral nutrition. National Institute for Health and Care Excellence. 2020. Accessed May 2025. https://www.nice.org.uk/guidance/NG154
- 20.Guidelines for Newborn Blood Spot Sampling. NHS Screening Programmes. 2015. Accessed May 2025. https://assets.publishing.service.gov.uk/media/5a7f91a240f0b62305b87f05/Guidelines_for_Newborn_Blood_Spot_Sampling_2015_v0_5.pdf#:~:text=The%20blood%20spot%20sample%20should%20be%20taken%20on,systems%20record%20day%20of%20birth%20as%20day%201%29
- 21.Matte GS, Kwon M, Mayer JE Jr. Fundamentals of cardiopulmonary bypass for congenital heart surgery. Nadas’ Pediatric Cardiology. Published online 2025:719-730. doi:10.1016/b978-1-4557-0599-3.00069-7
- 22.Flucloxacillin. BNF for Children. Accessed May 2025. https://bnfc.nice.org.uk/drugs/flucloxacillin/
- 23.Milrinone 1mg/mL solution for injection/infusion. Electronic Medicines Compendium. 2023. Accessed May 2025. https://www.medicines.org.uk/emc/product/2625/smpc
- 24.Dopamine hydrochloride 40mg/mL concentrate for solution for infusion. Electronic Medicines Compendium. 2023. Accessed May 2025. https://www.medicines.org.uk/emc/product/6619/smpc
- 25.BNF for Children. BNF for Children. Accessed May 2025. https://bnfc.nice.org.uk
- 26.Aspirin. BNF for Children. Accessed May 2025. https://bnfc.nice.org.uk/drugs/aspirin
- 27.Balloon angioplasty with or without stenting for coarctation or recoarctation of the aorta in adults and children. National Institute for Health and Care Excellence. July 2004. Accessed May 2025. https://www.nice.org.uk/guidance/ipg74/chapter/2-The-procedure
- 28.Endocarditis. British Heart Foundation. Accessed May 2025. https://www.bhf.org.uk/informationsupport/conditions/endocarditis
- 29.Endocarditis. NHS . 2022. Accessed May 2025. https://www.nhs.uk/conditions/endocarditis/
- 30.Amlodipine. BNF for Children. Accessed May 2025. https://bnfc.nice.org.uk/drugs/amlodipine
- 31.NHS Injectable Medicines Guide. Medusa. Accessed May 2025. https://www.medusaimg.nhs.uk/
- 32.The use of unlicensed medicines or licensed medicines for unlicensed applications in paediatric practice. Royal College of Paediatrics and Child Health. Accessed May 2025. https://www.rcpch.ac.uk/resources/use-unlicensed-medicines-or-licensed-medicines-unlicensed-applications-paediatric
- 33.Neonatal and Paediatric Pharmacists group. Position statement 2020-01 Choosing an Oral Liquid Medicine for Children. Royal College of Paediatrics and Child Health. 2020. Accessed May 2025. https://nppg.org.uk/wp-content/uploads/2020/12/Position-Statement-Liquid-Choice-V1-November-2020.pdf
- 34.Neonatal and Paediatric Pharmacists Group. Royal College of Paediatrics and Child Health. 2024. Accessed May 2025. https://nppg.org.uk/wp-content/uploads/2024/12/NPPG-Position-Statement-Standardised-Oral-Liquid-Concentrations-V10-December2024.pdf
- 35.Medicines for Children. Medicines for Children. Accessed May 2025. https://www.medicinesforchildren.org.uk/
- 36.Reye’s syndrome. NHS. 2023. Accessed May 2025. https://www.nhs.uk/conditions/reyes-syndrome/
- 37.Aspirin for prevention of blood clots. Medicines for Children. Accessed May 2025. https://www.medicinesforchildren.org.uk/medicines/aspirin-for-prevention-of-blood-clots/
- 38.Lim E, Parker E, Vasey N. Why learning how to swallow pills is good for patients, parents, and the planet. BMJ. Published online January 9, 2024:e076257. doi:10.1136/bmj-2023-076257
- 39.Kidzmet. E-Learning for Healthcare. Accessed May 2025. https://www.e-lfh.org.uk/programmes/kidzmed/
- 40.Slazak E, Shaver A, Clark CM, et al. Implementation of a Pharmacist-Led Transitions of Care Program within a Primary Care Practice: A Two-Phase Pilot Study. Pharmacy. 2020;8(1):4. doi:10.3390/pharmacy8010004
- 41.Yahya F, Nazar H, Huckerby C, Hadi MA. Facilitating the transfer of care from secondary to primary care: a scoping review to understand the role of pharmacists in general practice. Int J Clin Pharm. 2023;45(3):587-603. doi:10.1007/s11096-023-01547-3