Shutterstock.com
After reading this article, you should be able to:
- Understand the presenting symptoms of diabetic ketoacidosis (DKA);
- Understand the risk factors and prevention measures to assist people living with diabetes who are at risk of DKA;
- Understand the role of pharmacists and pharmacy technicians in prevention and treatment of DKA.
Diabetic ketoacidosis (DKA) is a disordered metabolic state resulting from the combination of absolute or relative insulin deficiency with increased production of counter-regulatory hormones[1]. Most episodes of DKA occur in people who have type 1 diabetes mellitus (T1DM); however, those with type 2 diabetes mellitus (T2DM) can experience DKA following catabolic stress (e.g. surgery, trauma and acute illness)[2].
DKA usually presents with the triad of hyperglycaemia, raised ketones (i.e. water-soluble molecules produced by gluconeogenesis in the liver) and acidosis (i.e. increased acidity in the blood and tissues); however, there is variability in its presentation.
DKA was previously only seen in T1DM patients, but with an increase in T2DM patients being given sodium-glucose transport protein 2 (SGLT2) inhibitors and more T2DM patients suffering with insulin resistance, up to a third of DKA cases are now seen in T2DM[2]. The cause of DKA in patients taking SGLT2 inhibitors is unclear but the increased incidence of dehydration and infections, both of which are risk factors for DKA, while taking this medication are implicated[3].
A type of DKA seen in T2DM is called ‘ketosis prone diabetes’, ‘flatbush diabetes’ or ‘type 1.5’. Treatment is the same, but often once treated the person will be able to cease insulin therapy. Mechanisms for this are still unknown and the clinical course is most similar to T2DM, with people often being islet/GAD antibody negative with no clear precipitating factors. It is more common in people who are of Afro-Caribbean or Hispanic descent[4].
In the UK, the incidence of DKA is between 8.0–51.3 cases per 1,000 T1DM patients and the prevalence is highest in patients aged 18-24 years[4]. DKA is costly to the NHS, with one study calculating the average cost of an episode at £2,064[5].
DKA is the leading cause of death among young–middle aged adults and children with T1DM[6]. Familiarisation with the symptoms of hyperglycaemia and DKA could help prevent morbidity and mortality associated with this serious acute metabolic complication[4].
The management of this medical emergency is complex and may require specialist input; however, each healthcare professional that cares for people living with diabetes has a responsibility for safety netting and the recognition of red flag symptoms.
Pathophysiology
In DKA, significantly reduced insulin concentrations and an increase in counter-regulatory hormones (catecholamines, cortisol, glucagon and growth hormones), causes hyperglycaemia with ketosis. Hyperglycaemia is driven by increases in hepatic gluconeogenesis and glycogenolysis, paired with a decrease in uptake of glucose into the peripheral tissues, as shown in Figure 1. The hormone imbalance and free fatty acids also drive resistance to any remaining insulin. The rise in blood glucose levels leads to osmotic diuresis causing significant dehydration and loss of electrolytes[7–12].
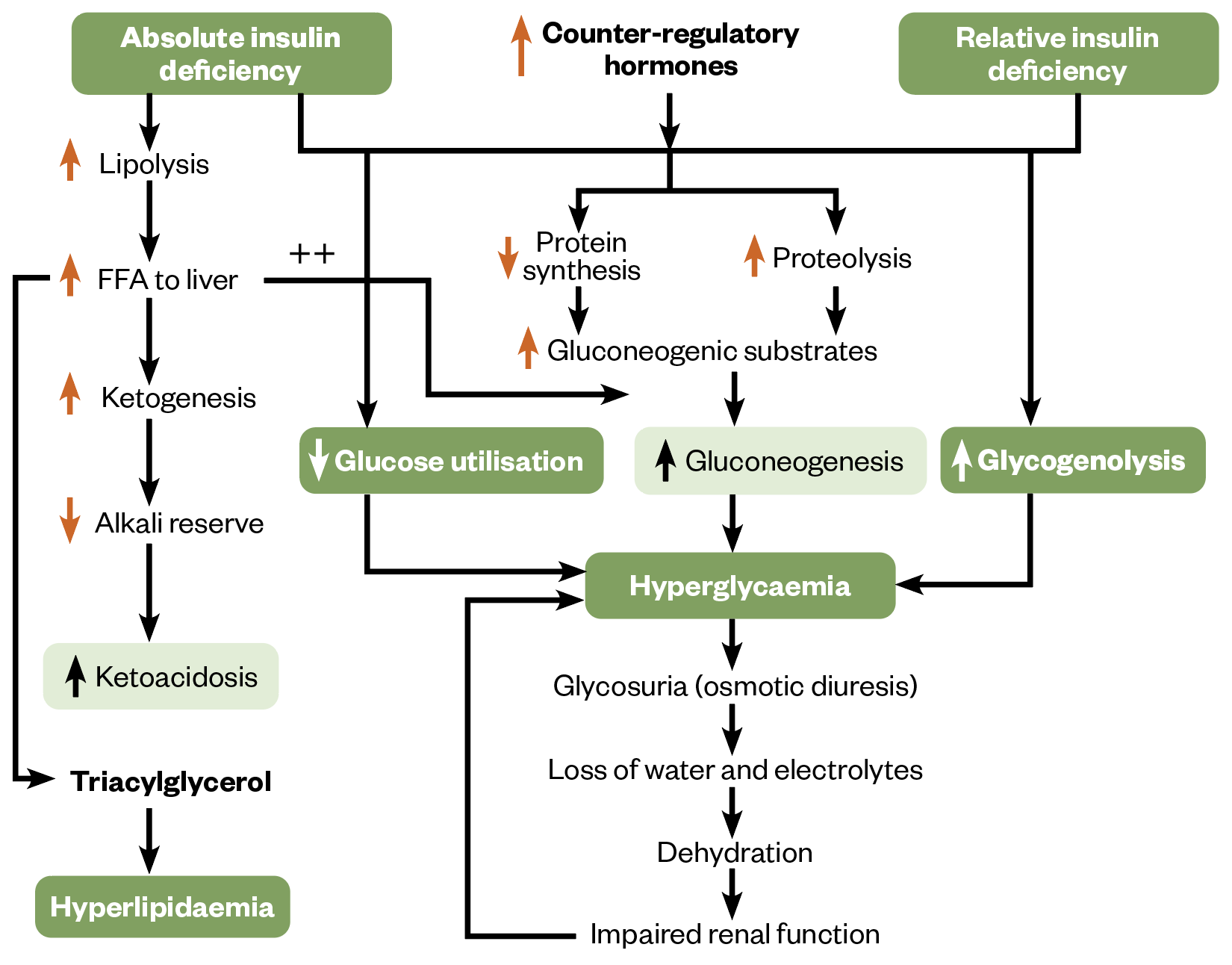
FFA: free fatty acids
The Pharmaceutical Journal
The insulin deficiency and counterregulatory hormones lead to the release of free fatty acids from adipose tissues. These are oxidised in the liver to ketone bodies. It is the build-up of these ketone bodies that leads to the metabolic acidosis seen in DKA[13].
Symptoms
The onset of DKA tends to be acute in nature. Symptoms of hyperglycaemia may present for several days and this may be followed by an acute onset (<24 hours) of symptoms suggestive of metabolic acidosis. Symptoms of hyperglycaemia can be seen in Box 1[14].
Box 1: The four ‘Ts’
Toilet — increased frequency of urination;
Thirsty — unable to quench thirst;
Tired — more tired than usual and no obvious causative factors for tiredness;
Thinner — losing weight without trying.
DKA symptoms include:
- Abdominal pain;
- Vomiting/nausea;
- Dehydration and poor skin turgor;
- Weakness;
- Altered mental state (e.g. confusion, black outs);
- Kussmaul respirations (deep, rapid breathing);
- Tachycardia;
- Hypotension;
- Hypothermia (peripherally vasodilated);
- Sweet smelling breath (fruity, ‘pear drops’)[15].
Some patients may only present with some of the listed symptoms. If a patient is suffering with these any of these symptoms, they should check their ketones and blood glucose to aid diagnosis[7].
Risk factors
The most common precipitator for DKA is infection, with UTI and pneumonia being the most common[16]. This is caused by increased insulin resistance owing to the increase in counter-regulatory hormones (cortisol and glucagon) during infection[17].
Other precipitating factors include discontinuation of insulin therapy or inadequate therapy, acute cardiovascular events (e.g. stroke, myocardial infarction), pancreatitis, and drugs that affect carbohydrate metabolism (e.g. corticosteroids, thiazide diuretics, antipsychotics, sympathomimetics)[7,8].
In young people with T1DM, there is a high prevalence of DKA in adolescence and 20% of recurrent DKA is linked to psychological wellbeing. There is a strong link with eating disorders, where insulin avoidance is used to avoid weight gain associated with therapy. It is estimated that in those aged between 15–30 years, 4 in 10 women and 1 in 10 men will engage in this behaviour[18].
Other factors that can lead to paused or reduced insulin administration in young people are fear of hypoglycaemia, diabetes distress (the emotional distress caused by living with diabetes and a need to rebel[7,8,19].
SGLT2 inhibitors can cause euglycemic diabetic ketoacidosis. A proposed mechanism, as detailed in Figure 2, is profound glucosuria (i.e. glucose in the urine), leading to decreased plasma glucose levels and decreased insulin release. Carbohydrate deficit, insulinopenia (i.e. low insulin) and increased glucagon release lead to upregulation of lipolysis and ketogenesis[20]. Normoglycaemia is caused by decreased carbohydrate intake and/or a deficit related to glucosuria[16].
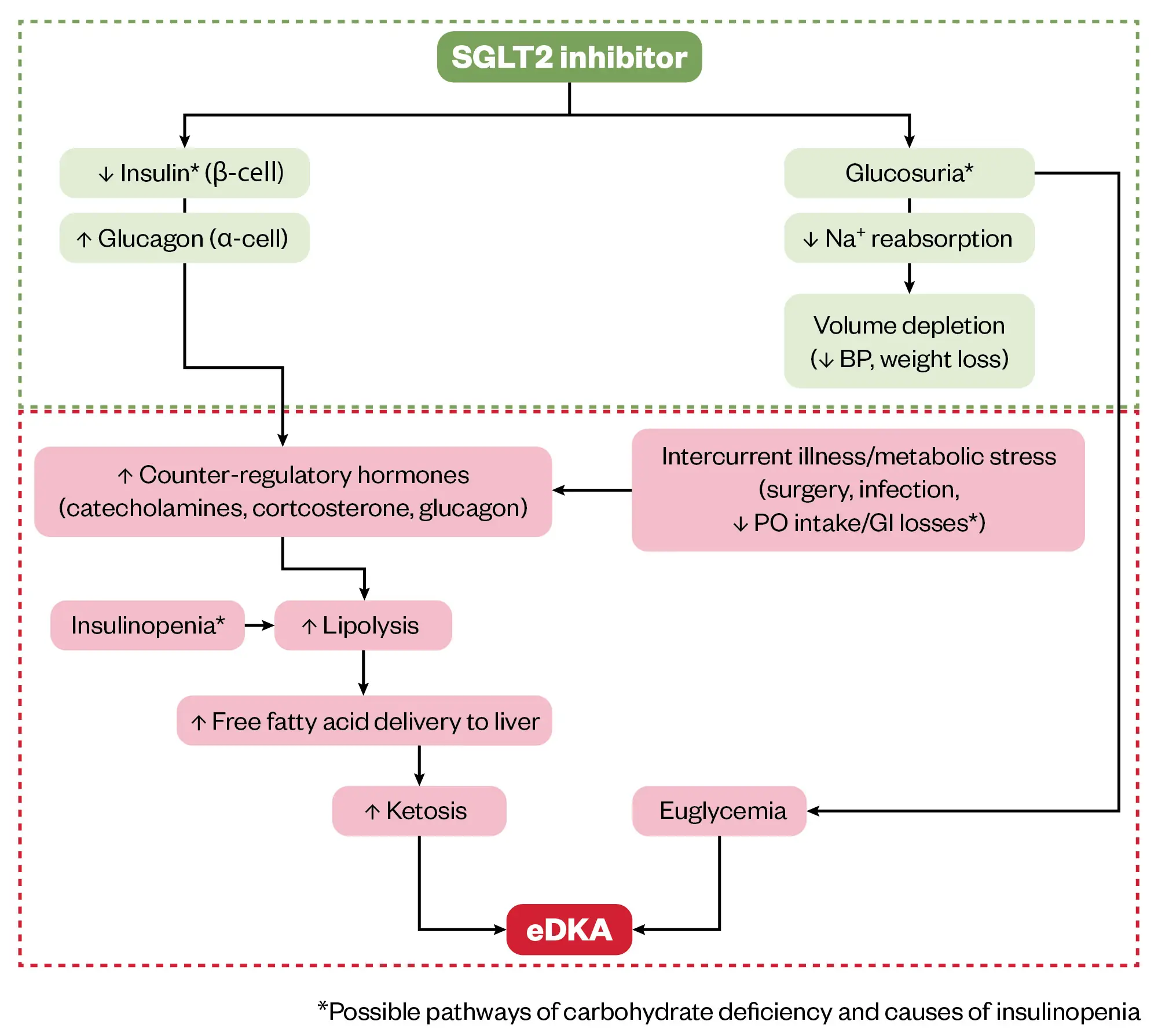
Proposed role of sodium-glucose cotransporter 2 (SGLT2) inhibition in euglycemic diabetic ketoacidosis (eDKA). Classic DKA results from insulin deficiency (absolute or relative) and concurrent increase in counter-regulatory hormones leading to ketosis, hyperglycemia, and osmotic diuresis. In contrast, SGLT2 inhibitor therapy in a well-compensated individual at baseline causes glucosuria, mild volume depletion, and lower serum glucose levels, associated with decreased insulin secretion (green box). During times of intercurrent illness and/or metabolic stress (eg, surgery or gastrointestinal illness), decreased carbohydrate intake coupled with lower serum glucose levels can further depress insulin secretion. This can ultimately lead to eDKA (red box).
Abbreviations: BP, blood pressure; PO, oral.
The Pharmaceutical Journal
Diagnosis
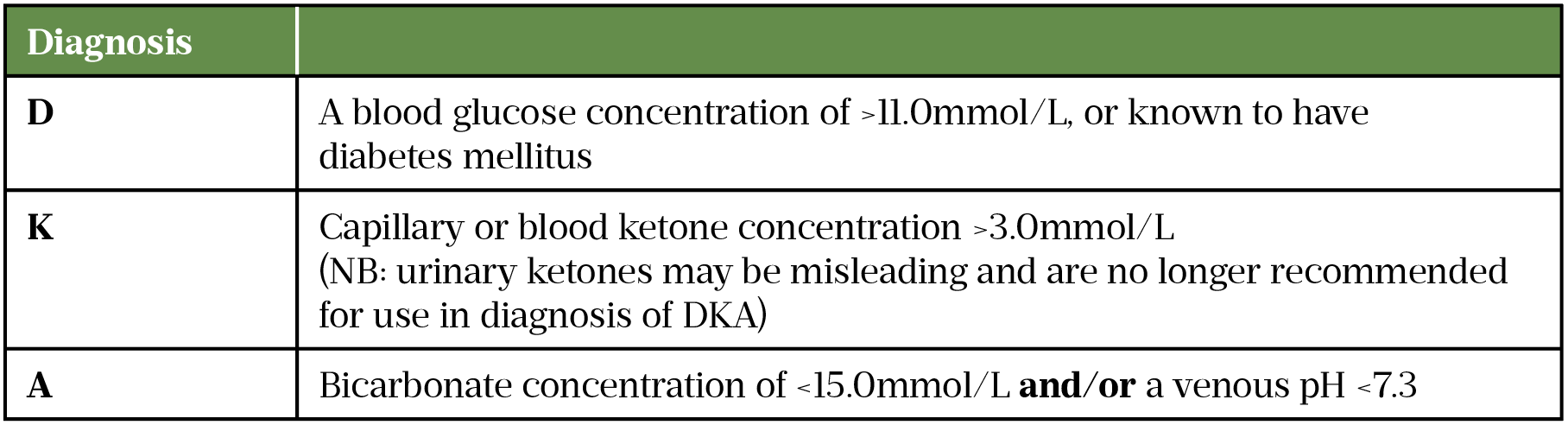
If a patient has symptoms of DKA they will need an urgent referral to A&E for treatment. The urgent care medical team will request investigations that include capillary blood glucose, blood ketones and arterial blood gases. Table 1 details the diagnostic criteria[21,22].
Intravenous (IV) access needs to be established and an assessment of ABC (airway, breathing, circulation), respiration rates, temperature, blood pressure, oxygen saturations and alertness (measured using the Glasgow Coma Scale) will help ensure the person is treated appropriately[23]. If IV access cannot be obtained, critical care support should be requested immediately. Nasogastric tubes may be situated to protect from aspiration[21,22]. Thromboprophylaxis with a low molecular weight heparin should be given to all patients unless contraindicated.
Initial investigations would include blood ketones, capillary blood glucose, venous plasma glucose, urea and electrolytes (including phosphate ideally), venous blood gases, full blood count and blood cultures. Some of these will be used for diagnostic purposes (ketones, venous blood gases, capillary blood glucose) and some will be used to guide therapy if a diagnosis is made (urea and electrolytes, capillary blood glucose)[21,22].
It is important to resolve the cause of the DKA to prevent recurrence or failure of resolution; therefore, it is advised to perform an ECG, chest X-ray if indicated, urinalysis and cultures, cardiac monitoring, pulse oximetry, pregnancy test for those of childbearing age, COVID-19/influenza testing (depending on the season and local procedures) and complete a thorough medication history to establish the patient’s usual medicines for diabetes[21,22].
Differential diagnosis
Clinical history and plasma glucose concentrations will help to distinguish between DKA and other forms of ketosis.
Starvation and alcoholic ketosis will present with a range of blood glucose concentrations from mildly elevated to hypoglycaemia (this is the main differing factor from DKA). However, a careful history is required to differentiate from euglycaemic DKA.
Starvation ketosis occurs owing to a lack of carbohydrates in the diet and develops over several days. This leads to ketosis and lipolysis and will often not be associated with profound acidosis[7].
Lactic acidosis (causes can include sepsis, liver impairment, hypothermia, hypervolemia, alcohol, metformin) should be ruled out if someone presents with suspected DKA. Lactic acidosis is more common in people with diabetes and in those who are hypovolaemic. It is good practice to measure plasma lactate on admission. This can be differentiated from DKA owing to the elevated lactate[7].
Ketosis can also be precipitated by overdose or substance abuse (e.g. salicylate, methanol, ethylene glycol and paraldehyde). There is some evidence to suggest that cocaine use may precipitate ketosis[24].
Other chronic conditions that may precipitate ketosis include acute chronic renal failure[7]. In addition, there have been case reports implicating endocrine disorders (i.e. pituitary gigantism and acromegaly)[25,26].
Management
Fluid replacement
There is universal agreement that the most important initial therapeutic intervention in DKA is fluid replacement followed by insulin administration.
The aims of fluid replacement are:
- Restoration of circulatory volume;
- Clearance of ketones;
- Correction of electrolyte imbalance.
In DKA, the intravascular, interstitial and intracellular volumes are all reduced and renal perfusion needs to be restored[27].
Immediate management involves the commencement of crystalloid, sodium chloride 0.9%, prior to starting the fixed rate insulin infusion (FRII), see below. A fluid bolus of 500mL over 10–15 minutes is given if the systolic blood pressure (SBP) is <90mmHg (with consideration given to age, gender, concomitant medication, heart failure, sepsis). This can be repeated if needed and care may be escalated (requiring a senior diabetologist and/or ICU/HDU involvement) if there is no improvement[21,28].
If the SBP is >90mmHg, 1L sodium chloride 0.9% IV is given over one hour.
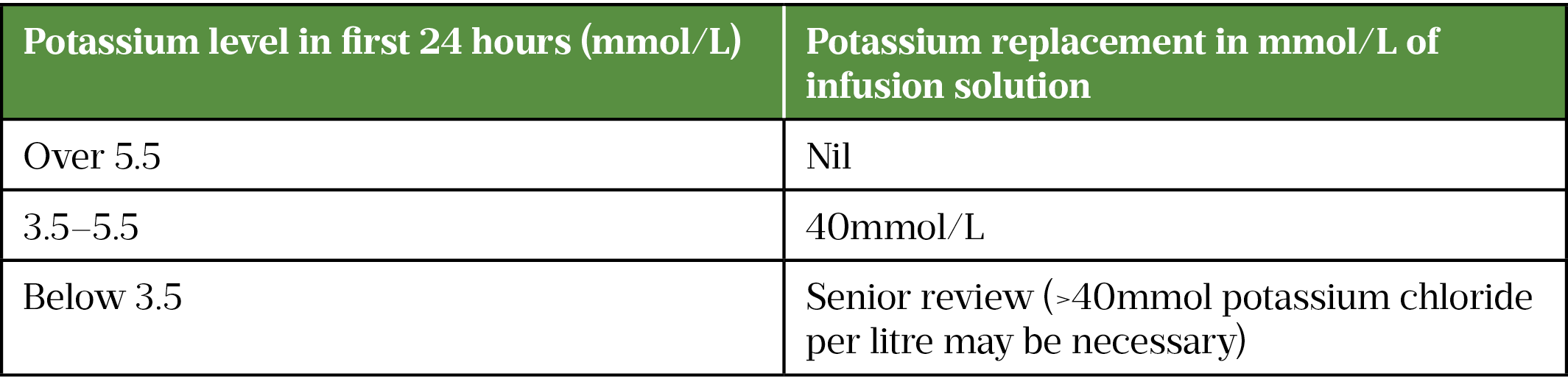
Potassium is then added to the second litre of IV fluid if potassium is <5.5 mmol/L; this may be needed in the first litre if more than one bag is required for initial fluid resuscitation[21,28].
A typical regimen for a 70kg adult patient with no comorbidities can be seen in Box 2[21]. Significant alterations and admission to (or at least discussion with) a high dependency unit may be needed for certain populations (e.g. young people aged 18–25 years, older people, pregnant, heart or renal failure and other significant comorbidities).
In pregnant women, a more cautious approach to fluid replacement is recommended and consideration for central line and admittance to a high dependency unit or setting is advised[21]. Foetal mortality can range from 9–36% and effects are often owing to maternal dehydration[29].
Box 2: Ongoing fluid replacement with normal saline
Volume of normal saline (with potassium chloride as needed):
- 1 litre over two hours;
- 1 litre over next two hours;
- 1 litre over next four hours;
- 1 litre over next four hours;
- 1 litre over next six hours.
Potassium replacement
Individuals with DKA often present with hyperkalaemia (potassium >5.5 mmol/L), which falls dramatically when insulin infusion is initiated[15]. Regular monitoring is essential to avoid life-threatening complications relating to potassium (including sinus bradycardia, cardiac conduction disturbances and neuromuscular function disturbances)[30].
Venous blood gases for pH, bicarbonate and potassium should be measured every hour for 2 hours, every 2 hours thereafter for up to 6 hours, and then 12-hourly after that (see Table 2[21].
Fixed rate intravenous insulin infusion therapy
Insulin acts to suppress ketone production, reduce blood glucose and correct electrolyte imbalance[21].
Fixed rate intravenous insulin infusion therapy (FRIII) is a solution of 50 units of human soluble insulin in 50mL 0.9% sodium chloride[21]. These can be procured ready-made, thus reducing infusion commencement time and the potential for errors in infusion preparation.
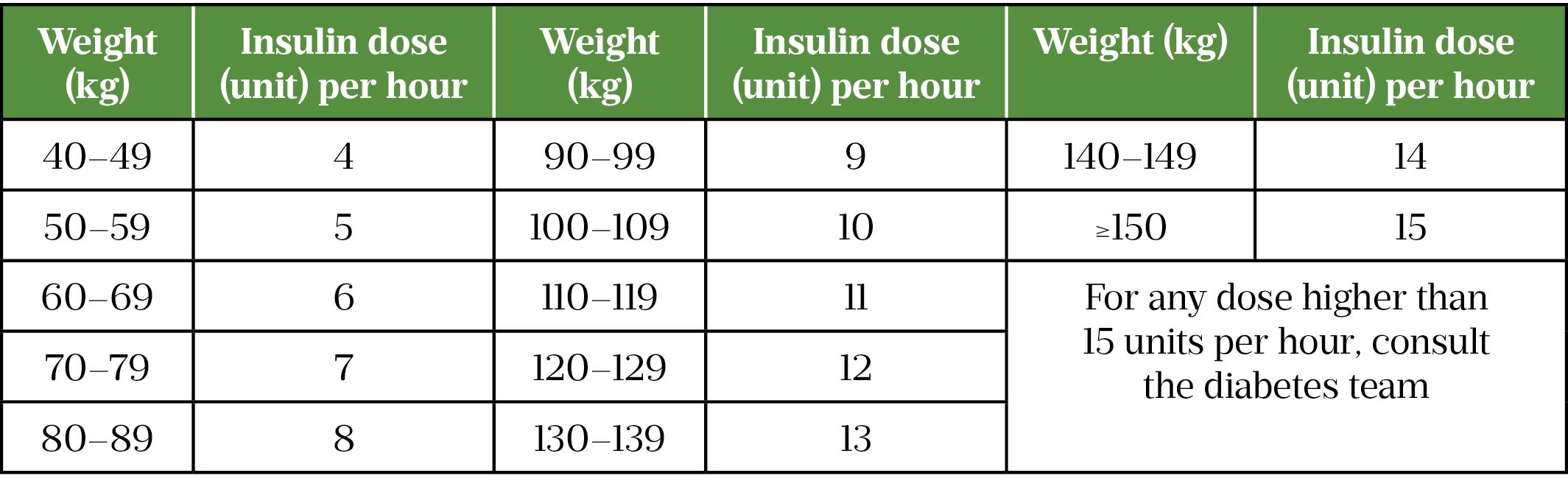
FRII is initially started at 0.1 units/kg, Table 3 details the initial infusion rates based on body weight. If weight is unable to be obtained, it should be estimated. In the case of pregnancy, the person’s actual weight should be used, and emergency obstetric support sought[21].
FRII should be continued until ketones are less than 0.6 mmol/L and venous pH is over 7.3. Venous bicarbonate should not be used as a marker of resolution, as the hypercholaemic acidosis associated with the large volumes of sodium chloride will lower bicarbonate levels[21].
Replacement of phosphate and bicarbonate
Bicarbonate maintains the pH balance is the body. Owing to the acidosis in DKA the bicarbonate level can be reduced[31]. Bicarbonate will correct as DKA resolves. Routine bicarbonate replacement is not indicated[31].
Routine phosphate replacement is not usually indicated; however, there are significant deficits during DKA (owing to transcellular shift and osmotic diureisis) that can worsen respiratory failure, rhabdomyolysis and cardiac arrhythmias[32]. Replacement should be considered in these scenarios using local guidance for phosphate replacement[21].
Glucose
When blood glucose is <14mmol/L, IV glucose 10% infusion should be introduced alongside sodium chloride 0.9% to prevent hypoglycaemia. This should not be discontinued until the patient is eating and drinking normally[21].
Monitoring
Continuous monitoring of ketones
Ketones should be monitored hourly and should fall by 0.5mmol/L/hr, if this is not the case, insulin infusion should be increased by 1.0 unit/hr; the FRIII pump should always be checked for proper function prior to this. In the absence of blood ketones, FRIII should be increased by 1.0 unit/hr as above if venous bicarbonate is not rising by at least 3.0mmol/L/hr[21].
Continuous monitoring of blood glucose
Hourly glucose readings should be taken. In the absence of ketones or venous bicarbonate, the infusion rate should be increased by 1.0unit/hr if blood glucose is not falling by at least 3.0mmol/L/hr. It should be noted that blood glucose does not indicate if ketosis is resolving[21].
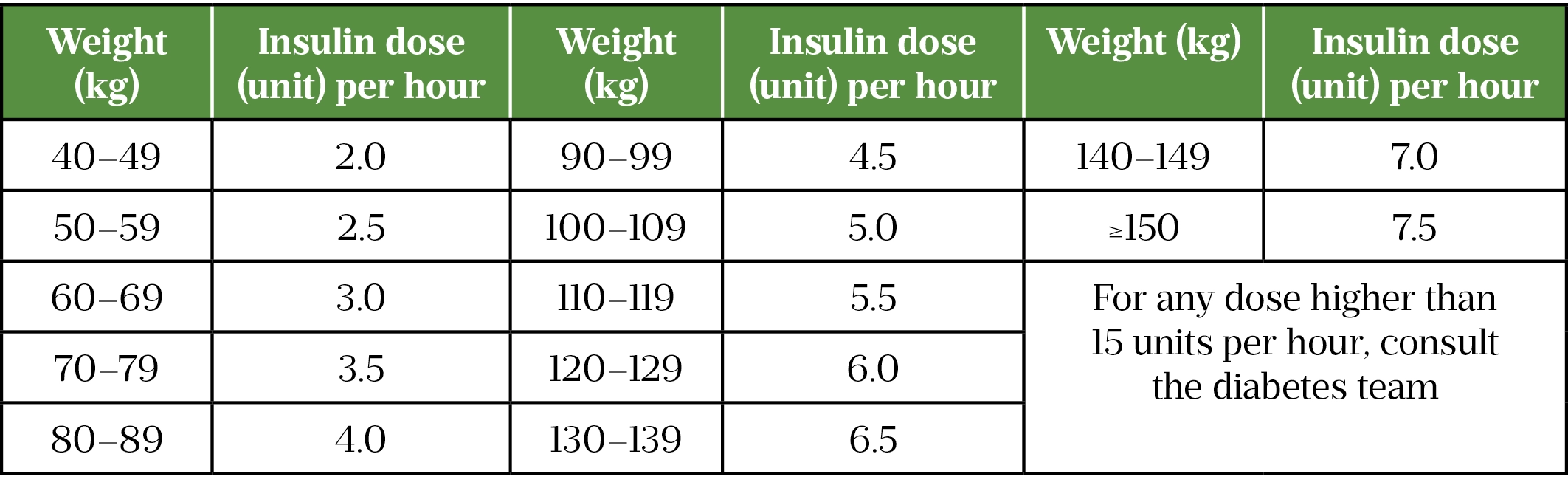
Once blood glucose drops below <14mmol/L, reducing the rate of insulin to 0.05 units/kg/hr should be considered to avoid risk of hypoglycaemia, as detailed in Table 4[21]. This is in addition to prescribing glucose 10% IV infusion[21]. Monitoring blood glucose levels every 1–2 hours can prevent hypoglycaemia as patients may not experience symptoms of hypoglycaemia[7,21].
Establishing long-term therapy and stopping IV insulin
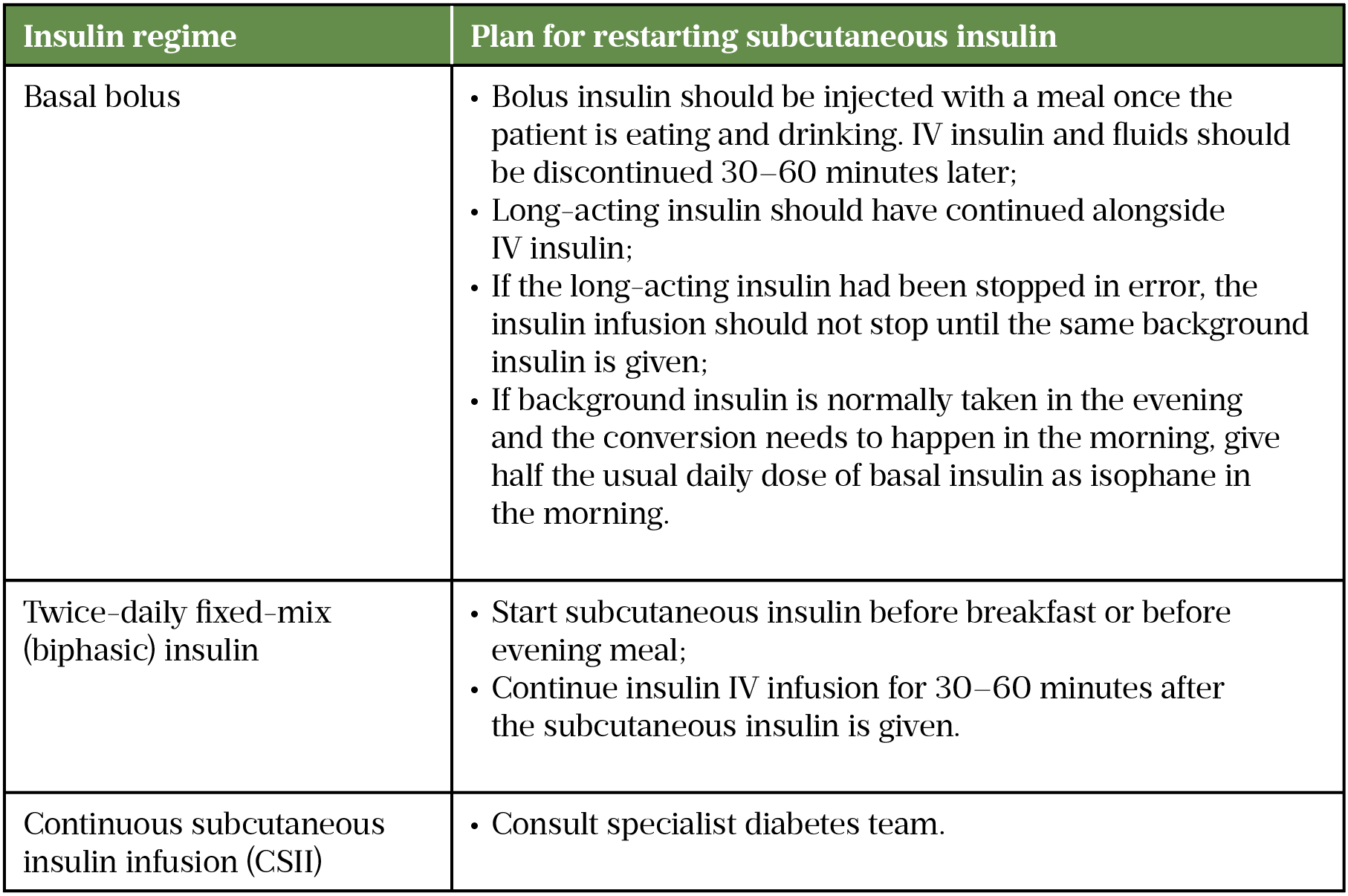
Re-establishing a person’s usual insulin regimen or initiating a long-term insulin regimen in those newly diagnosed, is usually done by the diabetes specialist team. It is good practice for secondary care settings to have this input seven days a week[21]. This is managed by referrals to the diabetes specialist team or patients moving to a specialist diabetes ward. Ideally, basal insulin should be continued as usual alongside FRIII, ensuing the basal insulin is continued alongside FRIII is a common and significant pharmaceutical intervention in the management of DKA. Table 5 details how to restart insulin in these cases[21].
Managing a patient’s long-term subcutaneous insulin is an important role for the pharmacist in the management of DKA.
Management in specific patient groups
Paediatrics
If a 16-18-year-old has their diabetes managed by an adult team rather than paediatrics, adult guidelines should be used[21]. Paediatric management is beyond the scope of this article, please refer to specific paediatric guidelines for more information[33].
Renal failure
Patients with end-stage renal failure can present with hyperglycaemia and ketosis. This group of patients are unable to develop an osmotic diuresis; therefore, fluid replacement may not be required as DKA can occur without much dehydration[21].
Insulin is the primary treatment and should be started at 0.1 units/kg/hr. It is advised to reduce to 0.05 units/kg/hr when glucose is <14mmol/L. Potassium is unlikely to be needed in a patient with renal failure owing to the lack of osmotic diuresis; however, hyperkalaemia is more common. Increased cardiac monitoring is advised owing to this potential complication[21].
Complications
Cerebral oedema
The cause of cerebral oedema in DKA is unknown. It often occurs in younger patients and if suspected should be treated urgently with mannitol or hypertonic saline to induce osmotic fluid shift. Treatment should not be delayed even if imaging is yet to occur. Symptoms include reduced Glasgow Coma Score, vomiting, incontinence, irritability and abnormal respirations[5].
Prevention
It is important to investigate the causative factors in an episode of DKA. This is often essential to prevent future occurrence.
A full review of diabetes care should be undertaken, including a review of ‘good injection technique’ (please see useful resources) and injection sites (for signs of lipohypertrophy, which may be impairing insulin absorption), abilities to self-care and provision of information to help people self-care in the future (e.g. provision of written advice on sick day rules and a written care plan)[21].
The person’s technology and insulin should be reviewed. Checking for any blood glucose monitor failures, pump failures, expired insulin and failing insulin pens. Ideally, someone who has had a DKA would have access to a ketone meter.
The person should be assessed for any mental health needs, particularly where repeat admissions for DKA have occurred.
Educating patients with diabetes on the importance of monitoring their blood glucose and ketones regularly has led to earlier interventions for treating and identifying DKA. Patients can be supported in this by having access to point of care testing and ensuring quality control of monitoring devices[21,28]. Pharmacists and pharmacy technicians can reinforce such education both in primary and secondary care.
Patients can be signposted to the Diabetes UK website for further resources and information regarding symptoms of DKA and monitoring. Local diabetes services should also be referred to.
Best practice
- DKA is a medical emergency. Recognition of the signs of symptoms of is essential to ensure treatment is received urgently;
- People living with diabetes who are at risk should be educated on self-care to avoid DKA and ensure they know where to access assistance if needed;
- People living with diabetes should also be educated on the importance of never discontinuing insulin therapy particularly when they are unwell;
- Ensure fluids are appropriately prescribed within local guidance and correct monitoring is adhered to;
- Involve diabetes specialist teams in establishing and reviewing insulin regimens after an episode of DKA;
- Establishing precipitating factor/s for DKA will help avoid any further occurrences;
- Ensure people who have had DKA can access a dual blood glucose and ketone meter and know when to measure ketones (e.g. when unwell/symptomatic).
Useful resources
- Joint British Diabetes Societies for Inpatient Care. ‘The management of diabetic ketoacidosis in adults‘.
- Joint British Diabetes Societies for inpatient care. ‘Diabetes at the front door‘.
- Diabetes UK. ‘What is diabetic ketoacidosis?’
- Forum for Injection Technique. FIT UK & FIT Ireland Forum for Injection Technique.
This article has been reviewed and updated by Claire Davies, diabetes and endocrinology specialist pharmacist, Gateshead Health NHS Foundation Trust, to ensure it remains relevant, following its original publication in March 2022.
- 1Clark ML, Kumar P. Kumar & Clark’s Clinical Medicine E-Book. Elsevier Health Sciences 2016.
- 2Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in Hospital Admission for Diabetic Ketoacidosis in Adults With Type 1 and Type 2 Diabetes in England, 1998–2013: A Retrospective Cohort Study. Dia Care. 2018;41:1870–7. doi:10.2337/dc17-1583
- 3Ata F, Yousaf Z, Khan AA, et al. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in a multicentric cohort. Sci Rep. 2021;11. doi:10.1038/s41598-021-89752-w
- 4Us, diabetes and a lot of facts and stats. Diabetes UK. 2019.https://www.diabetes.org.uk/resources-s3/2019-11/facts-stats-update-oct-2019.pdf (accessed Mar 2022).
- 5Dhatariya KK, Parsekar K, Skedgel C, et al. The cost of treating diabetic ketoacidosis in an adolescent population in the UK: a national survey of hospital resource use. Diabet. Med. 2019;36:982–7. doi:10.1111/dme.13893
- 6Gibb FW, Teoh WL, Graham J, et al. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia. 2016;59:2082–7. doi:10.1007/s00125-016-4034-0
- 7Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic Crises in Adult Patients With Diabetes. Diabetes Care. 2009;32:1335–43. doi:10.2337/dc09-9032
- 8Kitabchi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic Crises in Adult Patients With Diabetes. Diabetes Care. 2006;29:2739–48. doi:10.2337/dc06-9916
- 9Barrett EJ, DeFronzo RA, Bevilacqua S, et al. Insulin Resistance in Diabetic Ketoacidosis. Diabetes. 1982;31:923–8. doi:10.2337/diab.31.10.923
- 10Luzi L, Barrett EJ, Groop LC, et al. Metabolic Effects of Low-Dose Insulin Therapy on Glucose Metabolism in Diabetic Ketoacidosis. Diabetes. 1988;37:1470–7. doi:10.2337/diab.37.11.1470
- 11van de Werve G, Jeanrenaud B. Liver glycogen metabolism: An overview. Diabetes Metab. Rev. 1987;3:47–78. doi:10.1002/dmr.5610030104
- 12FELIG P, SHERWIN RS, SOMAN V, et al. Hormonal Interactions in the Regulation of Blood Glucose. Proceedings of the 1978 Laurentian Hormone Conference. 1979;:501–32. doi:10.1016/b978-0-12-571135-7.50016-3
- 13Miles JM, Haymond MW, Nissen SL, et al. Effects of free fatty acid availability, glucagon excess, and insulin deficiency on ketone body production in postabsorptive man. J. Clin. Invest. 1983;71:1554–61. doi:10.1172/jci110911
- 14Do you know the 4 Ts of type 1 diabetes? Diabetes UK. 2021.https://www.diabetes.org.uk/get_involved/campaigning/4-ts-campaign (accessed Mar 2022).
- 15What is DKA (diabetic ketoacidosis)? Diabetes UK. 2021.https://www.diabetes.org.uk/guide-to-diabetes/complications/diabetic_ketoacidosis (accessed Mar 2022).
- 16Umpierrez GE, Kitabchi AE. Diabetic Ketoacidosis. Treatments in Endocrinology. 2003;2:95–108. doi:10.2165/00024677-200302020-00003
- 17Affinati AH, Wallia A, Gianchandani RY. Severe hyperglycemia and insulin resistance in patients with SARS-CoV-2 infection: a report of two cases. Clin Diabetes Endocrinol. 2021;7. doi:10.1186/s40842-021-00121-y
- 18Diabulimia and diabetes. Diabetes UK. 2021.https://www.diabetes.org.uk/guide-to-diabetes/life-with-diabetes/diabulimia (accessed Mar 2022).
- 19Chapter 3 – diabetes distress. Diabetes UK. 2021.https://www.diabetes.org.uk/professionals/resources/shared-practice/psychological-care/emotional-health-professionals-guide/chapter-3-diabetes-distress (accessed Mar 2022).
- 20Milder DA, Milder TY, Kam PCA. Sodium-glucose co-transporter type-2 inhibitors: pharmacology and peri-operative considerations. Anaesthesia. 2018;73:1008–18. doi:10.1111/anae.14251
- 21The Management of Diabetic Ketoacidosis in Adults. Joint British Diabetes Societies for Inpatient Care . 2021.https://abcd.care/sites/abcd.care/files/site_uploads/JBDS_02%20_DKA_Guideline_amended_v2_June_2021.pdf (accessed Mar 2022).
- 22Dhatariya K, James J, Kong M ‐F., et al. Diabetes at the front door. A guideline for dealing with glucose related emergencies at the time of acute hospital admission from the Joint British Diabetes Society (JBDS) for Inpatient Care Group*. Diabet. Med. 2020;37:1578–89. doi:10.1111/dme.14304
- 23Jain S, Iverson L. statpearls. Published Online First: 20 June 2021.http://www.ncbi.nlm.nih.gov/books/NBK513298/
- 24Nyenwe EA, Loganathan RS, Blum S, et al. Active use of Cocaine: An Independent Risk Factor for Recurrent Diabetic Ketoacidosis in a City Hospital. Endocrine Practice. 2007;13:22–9. doi:10.4158/ep.13.1.22
- 25Soveid M, Ranjbar-Omrani G. Ketoacidosis As The Primary Manifestation Of Acromegaly. Arch Iran Med 2005;8:326–328.https://www.researchgate.net/publication/228791964_Ketoacidosis_as_the_primary_manifestation_of_acromegaly
- 26Kuzuya T, Matsuda A, Sakamoto Y, et al. A case of pituitary gigantism who had two episodes of diabetic ketoacidosis followed by complete recovery of diabetes. Endocrinol Jpn 1983;30:329–34. doi:10.1507/endocrj1954.30.329
- 27Hillman K. Fluid resuscitation in diabetic emergencies — a reappraisal. Intensive Care Med. 1987;13:4–8. doi:10.1007/bf00263548
- 28Karslioglu French E, Donihi AC, Korytkowski MT. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: review of acute decompensated diabetes in adult patients. BMJ. 2019;:l1114. doi:10.1136/bmj.l1114
- 29Mohan M, Baagar KAM, Lindow S. Management of diabetic ketoacidosis in pregnancy. Obstet Gynecol. 2017;19:55–62. doi:10.1111/tog.12344
- 30Viera A, Wouk N. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician 2015;92:487–95.https://www.ncbi.nlm.nih.gov/pubmed/26371733
- 31Chua HR, Schneider A, Bellomo R. Bicarbonate in diabetic ketoacidosis – a systematic review. Ann. Intensive Care. 2011;1. doi:10.1186/2110-5820-1-23
- 32van der Vaart A, Waanders F, van Beek AP, et al. Incidence and determinants of hypophosphatemia in diabetic ketoacidosis: an observational study. BMJ Open Diab Res Care. 2021;9:e002018. doi:10.1136/bmjdrc-2020-002018
- 33Heddy N. Guideline for the management of children and young people under the age of 18 years with diabetic ketoacidosis (British Society for Paediatric Endocrinology and Diabetes). Arch Dis Child Educ Pract Ed. 2021;:edpract-2020-320076. doi:10.1136/archdischild-2020-320076
1 comment
You must be logged in to post a comment.



Thorough and comprehensive. On a personal note; as a T1DM sufferer since 1974, many of the acute treatment options have not changed over the last 50 years. There were no such things as SGOT2 inhibitors back then and I just wonder whether they have muddied the waters somewhat for Luddite, old fogeys like myself.