Shutterstock.com
After reading this article, you should be able to:
- Understand the importance of the formulation and dosage of hydrocortisone in children;
- Assess the advantages and disadvantages of the various hydrocortisone options for specific patient groups;
- Offer useful advice and practical support to patients, parents and carers.
Hydrocortisone is a corticosteroid that is essential for the management of adrenal insufficiency, severe acute asthma and acute hypersensitivity reactions in paediatrics[1]. Careful dosing is required to avoid serious adverse effects and toxicity; however, long-term use poses several challenges for healthcare professionals and patients, particularly in relation to the choice of formulation and ease of administration of appropriate doses. There are four oral formulations of hydrocortisone available in the UK.
Pharmacists can support children, their parents and carers, and other healthcare professionals by advising on the safe use of an appropriate hydrocortisone formulation, including switching and providing counselling to help manage difficult dose administration at home. It is also important that patients, parents and carers can recognise the signs and symptoms of adrenal insufficiency[2,3]. Advice on prescribing alternative formulations is particularly important during temporary supply shortages, since missing doses carries significant risk of acute adrenal insufficiency[4].
This article outlines the important clinical and pharmaceutical considerations when recommending a hydrocortisone formulation, and the related counselling advice and practical support to provide patients.
Common uses
In children, hydrocortisone is most commonly used to treat primary adrenal insufficiency, which is caused by congenital adrenal hyperplasia (CAH) (70% of cases) and Addison’s disease (15% of cases)[5,6]. It is also used to manage secondary adrenal insufficiency caused by pituitary hormone deficiency.
CAH is a genetic disorder that requires high doses of hydrocortisone to replace deficient glucocorticoids and suppress overproduction of sex steroids. More than 90% of children with CAH will be prescribed hydrocortisone upon diagnosis, which is often continued for life[6].
The disorder affects around 1 in 15,000 children in the UK; however, the prevalence of Addison’s disease in children is less defined, but is around 1 in 10,000 for the whole population[6,7]. All children with Addison’s disease will require hydrocortisone (or another corticosteroid) throughout their life to replace deficient glucocorticoid production[6,7].
As these are long-term conditions managed with a high-risk drug, dose accuracy, patient acceptability and practicality of administration are paramount.
Choosing a formulation
When choosing or recommending a formulation, patient-specific factors and the properties of each formulation, alongside an assessment of advantages and disadvantages of each formulation, should be considered (see Table 1)[8,9].
![Table 1: Factors to consider when choosing an oral hydrocortisone formulation for children[8,9]](https://pharmaceutical-journal.com/wp-content/uploads/2021/11/Table-1-oral-hydrocortisone-factors-to-consider-1.png)
The ideal paediatric formulation should satisfy all desirable traits: high dose reproducibility, practicality, safety and low cost, as well as having a licensed status. However, it is unlikely that any one formulation will meet all criteria. A balance of benefits and risks should be made in relation to the child’s individual circumstances.
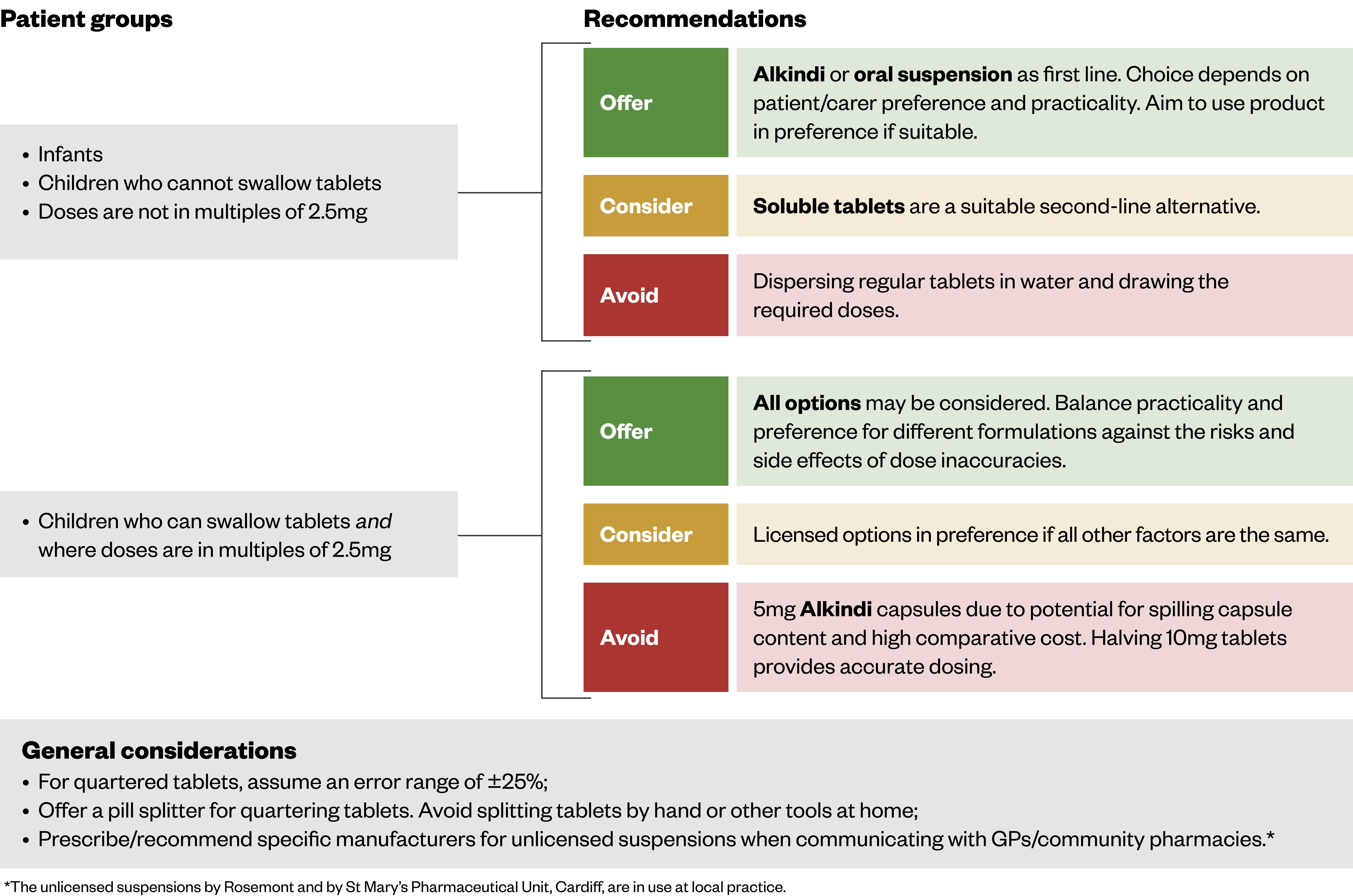
Although specific individual factors will influence the choice of formulation, pharmacists in secondary and tertiary care can help to standardise local practice by developing local guidance (see example in Figure 1). Local audits can be helpful to ensure guidance is being followed, and that formulations that are not recommended are not being used.
Dose accuracy
Excess dosing of hydrocortisone causes symptoms of cortisol excess (e.g. weight gain and stunted growth), while under-dosing is associated with symptoms of cortisol insufficiency, such as tiredness, nausea and poor concentration[10]. Abrupt withdrawal or interruption in dose after prolonged use can result in life-threatening acute adrenal insufficiency[3,4]. Symptoms can be non-specific (e.g. nausea, vomiting, fatigue, dizziness, hypotension and tachycardia) but can progress rapidly to coma and death[11]. Pharmacists should ensure children, and their parents and carers are aware of signs and symptoms of adrenal insufficiency, as infants and very young children are not able to communicate some of these symptoms, making them difficult to detect[10].
Adverse effects associated with under- or over-dosing of high-risk medicines can often be mitigated by regular monitoring and subsequent dose adjustment. However, this approach requires an available formulation of hydrocortisone that delivers consistent and reproducible doses to ensure that prescribed doses are delivered accurately. Children often require small doses of hydrocortisone, which can be difficult to achieve with some formulations — for example, tablets may need to be split or dispersed in water before drawing a specified volume of the resulting dispersion. This is a practice that produces highly variable results and can be difficult for children and their parents/carers to manage[12].
Children with adrenal insufficiency require daily doses of 8–10mg/m2 of hydrocortisone, while those with CAH require higher doses of 10–15mg/m2[11,13]. Doses slightly above this range (≥17mg/m2) have been shown to significantly diminish pubertal growth in children, highlighting the importance of dose accuracy and the need for accurate dose delivery[14]. This requires a formulation that delivers reproducible doses.
Understanding hydrocortisone formulations
Before 2018, hydrocortisone 2.5mg buccal tablets were sometimes used for infants and children owing to their convenient low strength and buccal dissolution. However, in 2018, the Medicines and Healthcare products Regulatory Agency (MHRA) issued a drug safety update, alerting healthcare professionals to the risk of insufficient systemic absorption and life-threatening adrenal crisis when using buccal tablets for adrenal replacement therapy in children[2]. The alert advised that prescribers and pharmacists should only consider use of licensed hydrocortisone products for adrenal replacement therapy and led practitioners to consider other formulations and methods of dose manipulation for children. This loss of the buccal formulation option was accompanied by the introduction of new formulations to the market, including soluble tablets and Alkindi granules (Diurnal), giving rise to questions about dose accuracy and practicality of each new formulation[15].
Tablets
Hydrocortisone tablets are available in strengths of 10mg and 20mg in the UK, with some 10mg tablets ‘quarter-scored’ to allow splitting to 2.5mg doses[16]. Although quartering these tablets is licensed, concerns have been highlighted around the reproducibility of doses, with as many as 54% of doses falling outside the 10% error margin[10]. The use of a tablet splitter produces better dose reproducibility than splitting by hand, even when scored tablets are used[17]. When a tablet splitter is used, scored and unscored tablets produce the same dose, although some children, parents and carers may be more comfortable with using scored tablets[12]. Unlike quartering tablets, halving the 10mg tablet to give 5mg doses appears to provide relatively consistent dosing[17]. Tablet hardness and friability also affect dose accuracy when splitting or dispersing them[18]. These properties are difficult to predict, since they vary with different manufacturers and even with the same manufacturer over time[18].
If the desired dose is not in multiples of 2.5mg, some practices advise dispersing the tablet in a small amount of water, followed by drawing the volume containing the desired dose. As tablets only form a coarse dispersion in water rather than dissolve, the particles tend to settle down quickly, and therefore obtaining uniform doses from the resulting dispersion can be challenging. A recent study showed that 57.1% of hydrocortisone doses prepared in this way fell outside a 20% error margin[12]. As a result, such practice is no longer recommended and other formulations would be more appropriate.
Soluble tablets
Hydrocortisone phosphate 10mg soluble tablets produce a true solution rather than a dispersion (see Figure 2)[19]. These tablets potentially offer more accurate dosing over tablets, although there are no solubility and dispersibility data available outside of the licensed recommendations. As a result, the manufacturer can only guarantee complete dissolution in at least 50mL of water and cannot advise on drawing part-doses from the resulting solution[19]. In practice, the tablets dissolve in as little as 10mL water, with a uniform physical appearance (see Figure 2). Despite the lack of data on true solubility and dispersibility, pharmacists should assess these cautions against dispersing regular (insoluble) tablets in water, which has demonstrated poor dose reproducibility in pharmaceutical studies[12].
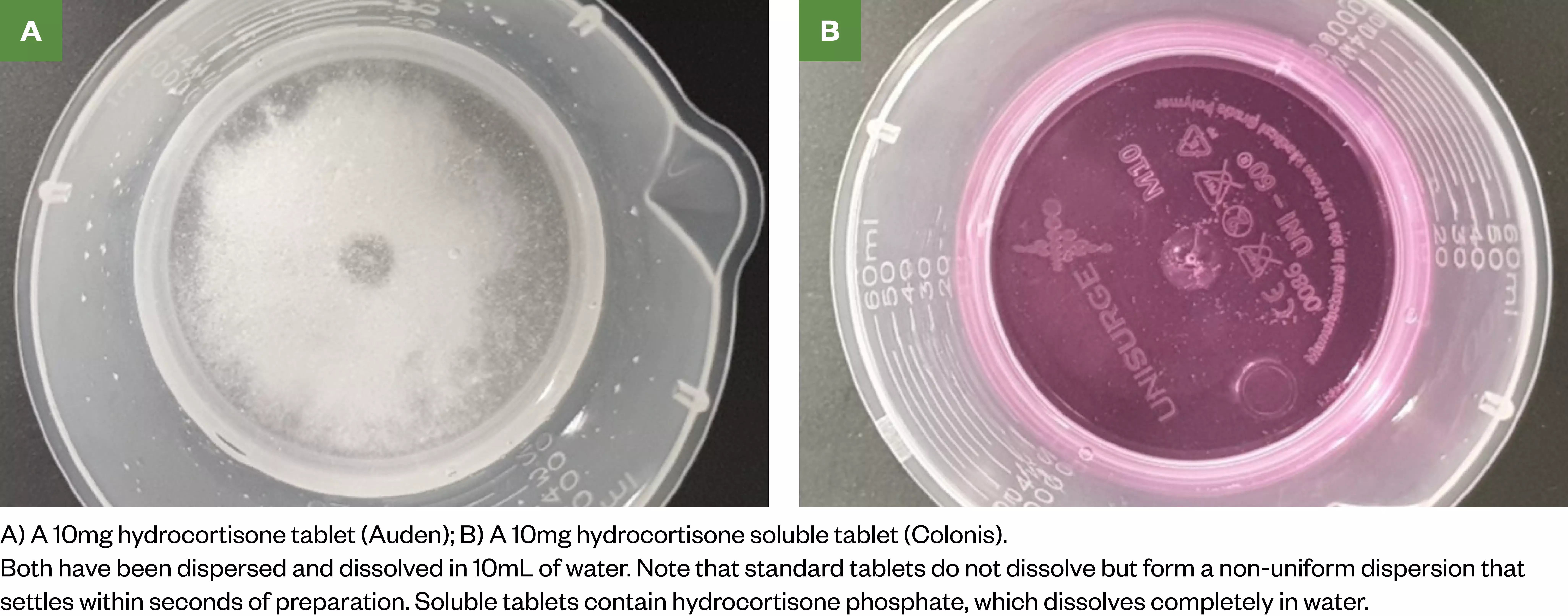
Images courtesy of Nabil Boulos
Oral suspensions
Hydrocortisone suspensions gained notoriety following loss of therapeutic control in children given a historical formulation in the late 1990s[20]. Hydrocortisone cypionate (Cortef oral suspension; Pfizer) was reformulated to change the suspending agent and add a cautionary label to shake the bottle for 5–7 minutes prior to use, given the inherent poor solubility. Despite this, the product was found to be non-bioequivalent to tablets and provided insufficient dosing, leading to its withdrawal from the market[20]. Many endocrinology textbooks still recommend against using hydrocortisone suspension; however, this historical example needs to be revised [21]. Many current suspensions no longer use the cypionate ester of hydrocortisone, but rather hydrocortisone acetate or micronised hydrocortisone base; a 2015 study demonstrated that newer suspensions provide doses bioequivalent to tablets, a conclusion that was also later acknowledged by the American Endocrine Society[13,21–23].
There are currently no licensed hydrocortisone suspensions available in the UK. Pharmacists should be aware that not all unlicensed products will be equivalent, and all efforts should be made to select reputable specials and unlicensed drug manufacturers when ordering and using hydrocortisone suspensions. Nevertheless, oral suspensions provide a suitable option for very small doses that can be given with fewer preparation steps (compared with soluble tablets) and that might be more familiar to parents and children (compared with Alkindi).
Granules in capsules
This novel formulation is licensed for children and provides an accurate and reproducible dosing option. It is available as 0.5mg, 1mg, 2mg and 5mg capsules and offers a suitable option for children who cannot swallow tablets or those requiring doses not in multiples of 2.5mg. Children need sufficient dexterity to open the capsule without spilling the granule content, which is an important consideration when encouraging children to gain more independence in managing their medicines. A demonstration of how to use this formulation is crucial when a patient is prescribed this for the first time. Cost is also a factor to consider, particularly for children on 5mg doses, which can otherwise be accurately obtained by halving a 10mg tablet.
The MHRA issued a drug safety update in February 2021, advising that parents or carers should be informed of the need to be extra vigilant for symptoms of adrenal insufficiency, such as tiredness, floppiness, unstable temperature, headache and vomiting, when switching children from hydrocortisone tablets to Alkindi granules[4].
Table 2 provides a comparison of hydrocortisone formulations using the factors discussed in Table 1.
![Table 2: Comparison of oral hydrocortisone formulations for paediatrics[12,13,15,17,19,21,22,24–26]](https://pharmaceutical-journal.com/wp-content/uploads/2021/11/Table-2-oral-hydrocortisone-comparison.png)
* Sodium benzoate can displace bilirubin from albumin in neonates, particularly those born preterm, potentially resulting in neonatal jaundice. Benzoate-containing formulations may be used with caution in this group. For other age groups, an acceptable maximum intake of benzoates is 5mg/kg/day.
Note: list of excipients and NHS indicative price for each formulation correct at time of publication.
Providing advice and practical support
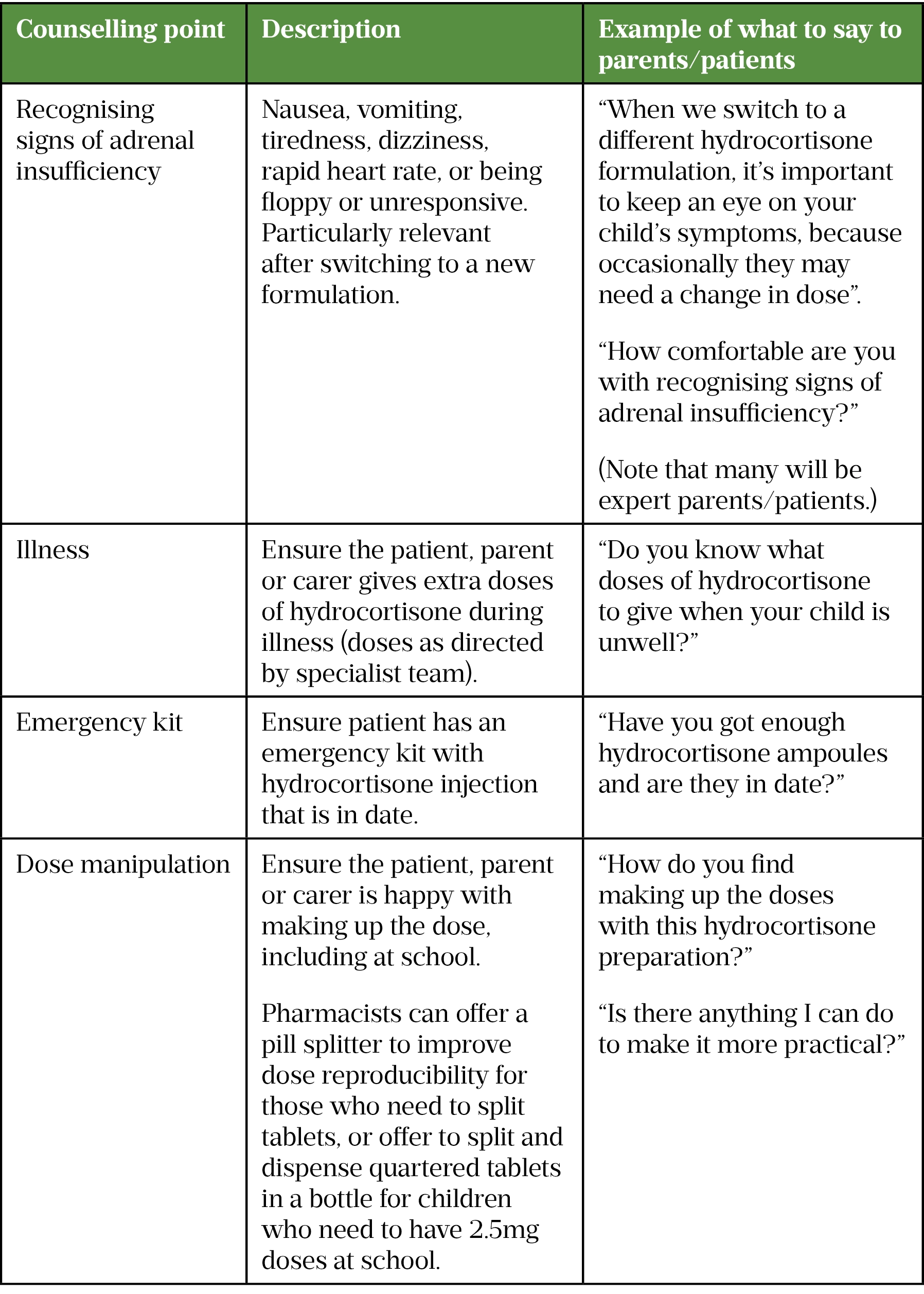
Many parents are not shown how to prepare medicine doses at the time of prescribing or require reminders. Pharmacists can offer to demonstrate how to prepare doses, although they should also be aware that some are ‘expert patients’ who are already confident with their methods through years of practice. Involving the child or young person in these discussions is encouraged whenever appropriate. Useful counselling points for patients starting hydrocortisone or switching formulations are shown in Table 3. More information on communicating with parents and involving children in medicines optimisation can be found here.
Summary
The expanding list of oral hydrocortisone formulations has prompted a review of formulation choices in paediatrics. Future formulations and strengths are already in development to meet the pharmaceutical and practical needs of this patient group, and undoubtedly present their own benefits and challenges. Assessment of dosing accuracy and reproducibility will still need to be considered alongside individual patient choice and practicality in everyday life. Pharmacists across sectors will continue to play a central role in this process, supporting patients and healthcare professionals with the safe use of hydrocortisone.
- 1British National Formulary for Children (online). MedicinesComplete. 2021.http://www.medicinescomplete.com (accessed Dec 2021).
- 2Hydrocortisone muco-adhesive buccal tablets: should not be used off-label for adrenal insufficiency in children due to serious risks, 2018. Medicines and Healthcare products Regulatory Agency. 2018.https://www.gov.uk/drug-safety-update/hydrocortisone-muco-adhesive-buccal-tablets-should-not-be-used-off-label-for-adrenal-insufficiency-in-children-due-to-serious-risks (accessed Dec 2021).
- 3Alkindi (hydrocortisone granules): risk of acute adrenal insufficiency in children when switching from hydrocortisone tablet formulations to granules. Medicines and Healthcare products Regulatory Agency. 2021.https://www.gov.uk/drug-safety-update/alkindi-hydrocortisone-granules-risk-of-acute-adrenal-insufficiency-in-children-when-switching-from-hydrocortisone-tablet-formulations-to-granules (accessed Dec 2021).
- 4National Patient Safety Alert: Steroid Emergency Card to support early recognition and treatment of adrenal crisis in adults. NHS England. 2020.https://www.england.nhs.uk/wp-content/uploads/2020/08/NPSA-Emergency-Steroid-Card-FINAL-2.3.pdf (accessed Dec 2021).
- 5Bowden SA, Henry R. Pediatric Adrenal Insufficiency: Diagnosis, Management, and New Therapies. International Journal of Pediatrics. 2018;2018:1–8. doi:10.1155/2018/1739831
- 6What is CAH? . Living with CAH. 2021.https://www.livingwithcah.com/about/ (accessed Dec 2021).
- 7What is Addison’s disease? Addison’s self-help group. 2021.https://www.addisonsdisease.org.uk/what-is-addisons-disease (accessed Dec 2021).
- 8Medicines and Healthcare products Regulatory Agency. The supply of unlicensed medicinal products (“specials”). 2014.https://www.gov.uk/government/publications/supply-unlicensed-medicinal-products-specials (accessed Dec 2021).
- 9The use of unlicensed medicines or licensed medicines for unlicensed applications in paediatric practice. Royal College of Paediatrics and Child Health. 2013.https://www.rcpch.ac.uk/resources/use-unlicensed-medicines-or-licensed-medicines-unlicensed-applications-paediatric (accessed Dec 2021).
- 10Madathilethu J, Roberts M, Peak M, et al. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. bmjpo. 2018;2:e000198. doi:10.1136/bmjpo-2017-000198
- 11Miller BS, Spencer SP, Geffner ME, et al. Emergency management of adrenal insufficiency in children: advocating for treatment options in outpatient and field settings. J Investig Med. 2019;68:16–25. doi:10.1136/jim-2019-000999
- 12Watson C, Webb EA, Kerr S, et al. How close is the dose? Manipulation of 10 mg hydrocortisone tablets to provide appropriate doses to children. International Journal of Pharmaceutics. 2018;545:57–63. doi:10.1016/j.ijpharm.2018.04.054
- 13Speiser PW, Arlt W, Auchus RJ, et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society* Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2018;103:4043–88. doi:10.1210/jc.2018-01865
- 14Bonfig W, Dalla Pozza SB, Schmidt H, et al. Hydrocortisone Dosing during Puberty in Patients with Classical Congenital Adrenal Hyperplasia: An Evidence-Based Recommendation. The Journal of Clinical Endocrinology & Metabolism. 2009;94:3882–8. doi:10.1210/jc.2009-0942
- 15Alkindi 0.5mg granules in capsules for opening. Electronic Medicines Compendium. 2021.https://www.medicines.org.uk/emc/product/9032/smpc (accessed Dec 2021).
- 16Hydrocortisone 10mg tablets. Electronic Medicines Compendium . 2020.https://www.medicines.org.uk/emc/product/5035/smpc (accessed Dec 2021).
- 17C Andersson Å, Lindemalm S, Eksborg S. Dividing the Tablets for Children – Good or Bad? PHME. 2016;7:23–7. doi:10.5530/phm.2016.7.4
- 18Saimbi S, Madden V, Stirling H, et al. COMPARISON OF HYDROCORTISONE 10 MG TABLETS: TABLET HARDNESS OPTIMISED FOR ADULT USE HAS NEGATIVE CONSEQUENCES FOR PAEDIATRIC USE. Arch Dis Child. 2016;101:e2.9-e2. doi:10.1136/archdischild-2016-311535.17
- 19Hydrocortisone 10mg soluble tablets. Electronic Medicines Compendium. 2021.https://www.medicines.org.uk/emc/product/11645 (accessed Dec 2021).
- 20Merke DP, Cho D, Anton Calis K, et al. Hydrocortisone Suspension and Hydrocortisone Tablets Are Not Bioequivalent in the Treatment of Children with Congenital Adrenal Hyperplasia. The Journal of Clinical Endocrinology & Metabolism. 2001;86:441–5. doi:10.1210/jcem.86.1.7275
- 21Sarafoglou K, Gonzalez-Bolanos MT, Zimmerman CL, et al. Comparison of cortisol exposures and pharmacodynamic adrenal steroid responses to hydrocortisone suspension vs. commercial tablets. The Journal of Clinical Pharmacology. 2014;55:452–7. doi:10.1002/jcph.424
- 22Hydrocortisone oral suspension. Rosemont Pharma. 2021.https://www.rosemontpharma.com/products/endocrine-system/hydrocortisone-oral-suspension (accessed Dec 2021).
- 23Hydrocortisone suspension 1mg in 1ml. St Mary’s Pharmaceutical Unit. 2021.https://www.smpu.co.uk/en/product/hydrocortisone-suspension-1mg-in-1ml (accessed Dec 2021).
- 24Daniel E, Whitaker MJ, Keevil B, et al. Accuracy of hydrocortisone dose administration via nasogastric tube. Clin Endocrinol. 2018;90:66–73. doi:10.1111/cen.13876
- 25Questions and answers on benzoic acid and benzoates in the context of the revision of the guidelines on ‘Excipients in the label and package leaflet of medicinal products for human use’. European Medicines Agency. 2014.http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500162031.pdf (accessed Dec 2021).
- 26Evaluation of certain food additives and contaminants. Forty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organ Tech Rep Ser 1997;868:i–viii, 1–69.https://www.ncbi.nlm.nih.gov/pubmed/9385867