
Shutterstock.com
After reading this article, you should be able to:
- Understand the prevalence of herbal medicine use during pregnancy;
- Identify the common indications and most frequently used herbal medicines during pregnancy globally;
- Explain the potential for herb–drug interactions;
- Understand how the limited information available can guide us on the use of herbal medicines commonly used during pregnancy.
The World Health Organization (WHO) defines herbal medicines as “herbs, herbal materials, herbal preparations and finished herbal products that contain as active ingredients, parts of plants, other plant materials or combinations”[1]. Herbal medicines span the spectrum from home-brewed teas prepared from collected leaves and herbs, to products with official approved status granted by national regulating authorities[2].
In the UK, herbal medicines are registered via the Traditional Herbal Registration (THR) Scheme under the Medicines and Healthcare products Regulatory Agency (MHRA), which requires herbal medicines to meet the specific and appropriate standards of safety and quality[3]. The authorised herbal medicine products can be found on the MHRA website[3]. Information on the safety of herbal medicines during pregnancy for more than 150 herbal products can be found in herbal monographs on the European Medicines Agency’s website[4].
A WHO survey published in 2019 recorded that 88% of WHO member states (170) have reported the use of traditional and complementary medicine[5]. In another report, it was estimated that 65–85% of the global population used herbal medicines as their primary form of healthcare[6]. Women have been identified as the main users of herbal medicines, and this widespread use extends into pregnancy[7,8].
With reference to several studies, it was reported that 10–74% of pregnant women in Africa, Australia, the United States, Europe and the UK use herbal medicines[9–19]. Prevalence depends on geographic location, ethnicity, cultural traditions and social status[20]. In the UK, approximately 40% of pregnant women use herbal medicines to treat pregnancy related problems or improve pregnancy outcomes[12,13]. The findings from eight cross-sectional studies (2,729 participants) conducted across seven Asian countries (data up to 2016) found 1,283 women (47% of the sample) reported that they had used one or more herbal medicine during pregnancy (See Figure 1[21]).
Another cohort study conducted in South West England found that 27% (n=3,774) of women had used complementary or alternative medicine at least once during pregnancy, with use increasing from 6% in the first trimester to 12% in the second, and to 26% in the third[22]. The use of complementary medicines also increased with the age of the mother, with the results showing that 44% of mothers aged above 35 years used complementary medicines, compared with 15% of respondents aged 24 years or younger[22].
Pregnant women may perceive herbal medicines as natural and safe alternatives to conventional medicines[23]. However, some plants have toxic constituents and many have constituents with pharmacological activity, such as stimulation of uterine muscle and induction of labour (e.g. black cohosh)[24,25]. Contamination with substances such as pesticides, conventional medicines or heavy metals (e.g. lead) also has to be taken into account[23]. For example, in 2012 there were reports of six cases of lead poisoning in New York resulting from the use of imported Ayurvedic herbal medicine by pregnant women[26].
Herbal medicine preparations often vary with respect to the concentration and origin of their constituent herbs[23,27]. Many modern herbal preparations are available as highly concentrated extracts and their effects could differ substantially from those of more traditional preparations, such as tea made from the herb’s leaves[23]. Therefore, it may be difficult to assess the safety aspect of the different preparations for a herbal medicine[23]. As with conventional drugs, there may be a number of formulations available — e.g. capsule, tinctures, cream, dried leaves — for a herbal medicine with different methods of administration[23]. This should be considered when determining the safety of herbal medicines as the rate of drug absorption may differ depending on the formulation used[23].
Indications for use of herbal medicines during pregnancy
The traditional indications for the use of herbal medicines during pregnancy can be related to the treatment of pregnancy-related problems or to improve the well-being of the mother or unborn child[7]. The commonly reported indications during pregnancy worldwide are[7]:
- Morning sickness;
- Cold and flu;
- Pain (gastralgia and other types of pain);
- Anxiety;
- Stress;
- Gastrointestinal disorders, such as constipation and flatulence;
- Oedema;
- Urinary tract infection;
- Labour preparation, facilitation and induction;
- Milk production and secretion;
- Foetal health promotion;
- Anaemia.
Pharmacological activity of different herbal medicines during pregnancy
The data from scientific studies into the effects of herbal medicines in pregnancy, and the literature reporting relating to the outcome of pregnancies during which herbal medicines were used, are relatively limited. In view of this, the use of herbal medicines during pregnancy is not recommended. Nevertheless, there is a large and growing number of pregnant women using herbal medicines and it is important for pharmacists to be able to communicate the available evidence to patients and to be able to work from first principles when considering why herbal medicines are not generally recommended during pregnancy[6]. The widespread use of herbal medicines has allowed for a number of cross-sectional studies to be conducted, which has produced some useful information to inform recommendations regarding some commonly used herbal medicines during pregnancy.
The boxes below provide examples of five herbal medicines, illustrating their indications for use and the supporting information underpinning recommendations around their use during pregnancy. Please note that this is a selection of examples to illustrate the limited nature of the information available and not an exhaustive list of herbal medicines.
Box 1: Blue cohosh
When used orally, blue cohosh is a uterine stimulant and can induce labour. Several blue cohosh constituents, such as anagyrine and N-methylcytisine, are potentially teratogenic and might cause congenital malformations in newborns.
Recommendation:
The potential foetal and newborn toxicity appears to outweigh any medical benefit. The use of blue cohosh should be avoided[28–33].
Box 2: Borage (Borage oil)
Borage is unsafe to be used in pregnancy when taken orally. This herbal medicine contains hepatotoxic pyrrolizidine alkaloids when it is used orally.
Recommendation:
This herbal medicine should be avoided during pregnancy owing to possible teratogenic effects. It has a prostaglandin E agonist action which can have labour inducing effects[30,33–35].
Box 3: Dong quai
One observational research study has found that intake of An-Tai-Yin, an herbal combination product containing dong quai and parsley, during the first trimester is associated with an increased risk of congenital malformations of the musculoskeletal system, connective tissue and eyes.
Recommendation:
Dong quai has uterine stimulant and relaxant effects and hence can potentially increase the risk of miscarriage. Dong quai is not recommended to be used during pregnancy[36–39].
Box 4: Ginger (root)
Between 50–80% of women experience nausea during pregnancy. A Cochrane review concluded that the use of ginger may be helpful to women, but the evidence of effectiveness was limited and not consistent. The current National Institute for Health and Care Excellence guidance on antenatal care mentions that ginger appears to be an effective intervention in reducing nausea and vomiting symptoms in early pregnancy.
According to information produced by the UK Teratology Information Service, results from three cohort studies, one case-control study and several small clinical trials showed no increase in the incidence of adverse pregnancy outcomes, including congenital malformations, when ginger was used during pregnancy. Despite this, since only small numbers of pregnant women have been exposed to ginger in studies, the risk of adverse effects cannot be completely ruled out.
Recommendation:
During pregnancy, it has been suggested that women do not take doses of ginger exceeding 1g/day for the indication of nausea and vomiting.
Ginger has limited potential to stimulate uterine smooth muscle. Despite this theoretical concern, the risk posed by ginger to the foetus is considered to be low, particularly when it is used at the doses found in foods, such as ginger biscuits[35,40–43].
Box 5: St John’s wort
St John’s wort induces CYP 3A4 and P-glycoprotein and therefore has the potential to interact with many drugs, including those conventional drugs taken by the pregnant women.
Preliminary population research has found that taking St. John’s wort while pregnant is associated with offspring that develop neural tube, urinary and cardiovascular malformation. Subgroup analyses suggest that these risks may be higher when taking St. John’s wort during the first trimester when compared with the second or third trimester. However, more research is needed to confirm these findings.
Animal-model research also shows that constituents of St. John’s wort might have teratogenic effects. Until more safety data is released, St. John’s wort should not be taken during pregnancy.
In the clinical guidelines on the management of depression in adults, NICE advises against prescribing or advising the use of St John’s wort; however, unintentional exposure to St John’s wort during early pregnancy may occur.
Recommendation:
In view of the lack of toxicity data, use of this herbal medicine during pregnancy is not recommended[28,44–47].
Herb–drug interactions
A further concern is herb–drug interactions between herbal medicines and any conventional medicine(s) being taken by a pregnant woman, which may potentially harm the mother and/or the foetus[8,48]. The number of women needing to take conventional medicines during pregnancy is increasing[49–52]. It is important that pharmacists are aware of any potentially harmful herb–drug interactions and educate patients around the risks.
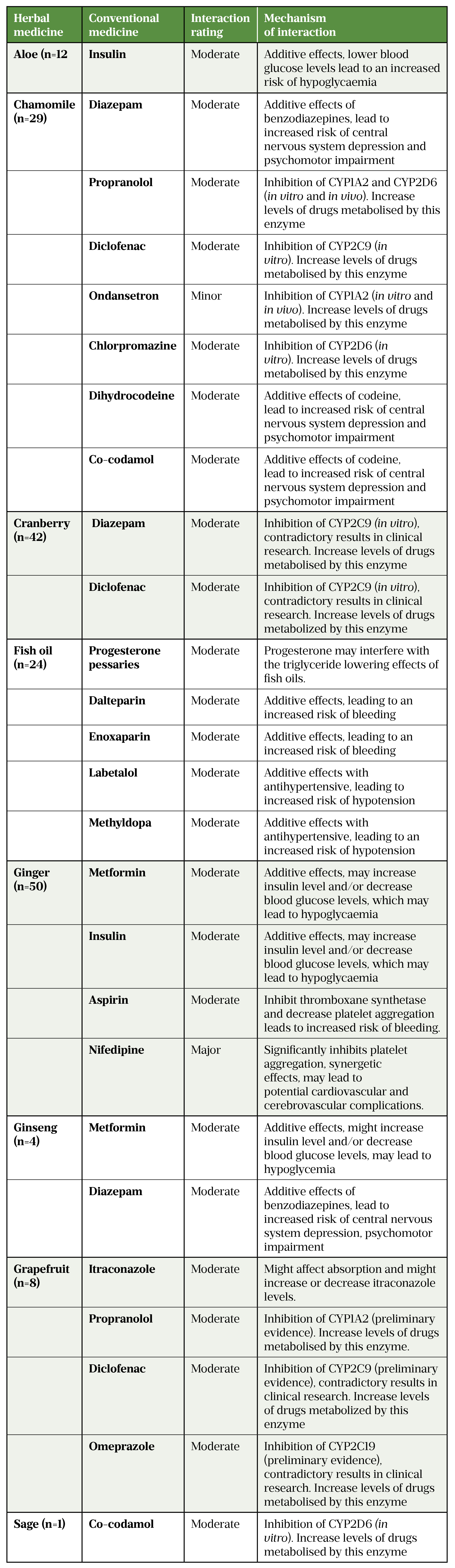
In a survey conducted in Scotland completed by 889 women during early pregnancy or immediately after delivery, 45% of women reported using prescription medicines (excluding vitamins)[48]. Of these respondents, 45% used at least one herbal medicine and a potential herb–drug interaction was identified in 13% (see Table[48]).
Practical advice in relation to the use of herbal medicines during pregnancy
Herbal medicines should generally be avoided during pregnancy as most herbal medicines have not undergone rigorous clinical testing before being made available. There might be little or nothing known about their therapeutic and adverse effects during pregnancy, as well as any drug–herbs interactions[23].
Women who wish to take herbal products medicinally during pregnancy should consult a healthcare professional, and the risks and benefits should be carefully assessed[23].
Healthcare professionals should consider the reason(s) why a woman wishes to take herbal medicine. Undiagnosed illness that remains untreated by conventional methods might result in maternal and foetal toxicity[23].
It is important for the healthcare professional to take a complete drug history, including the use of herbal medicines from the pregnant women both in the hospital and primary care setting[53,54].
Herbal medicines are not safe alternatives to conventional medicines during pregnancy. They might possess toxic constituents, contaminants and they may vary with regard to the concentration and source of their constituent herbs. Some herbal medicines may contain contaminants such as heavy metals, pesticides or conventional medicines, which may be harmful to the foetus[23]. It is important to counsel the mothers regarding this when they consider taking herbal medicines.
There is the potential for herbal medicines to interact with each other and with any conventional medicines being taken[23,48]. They can also pose problems in the peri-operative setting because of the drug interactions between the herbal medicine and anaesthesia; some are therefore recommended to stop two weeks prior to any surgery[24,55]. Ginseng, garlic and ginkgo for example may impair coagulation[23].
If herbal medicines are required as essential treatments, they should be obtained from a reputable source and taken at the recommended dosage[23].
Herbs that are commonly used in cooking would not be expected to be harmful during pregnancy in the quantities usually contained in foods; however, in large doses or concentrated forms, culinary herbs such as sage and garlic may be associated with risks in pregnancy[23,56].
Suspected adverse reactions (including congenital abnormalities) associated with maternal use of herbal medicines should be reported to the MHRA and the Commission on Human Medicines (CHM) via the yellow card reporting system[57].
Healthcare professionals can contact the UK Teratology Information Service (UKTIS) for assistance in making a patient-specific risk assessment when exposure to herbal medicines has occurred[58].
Other resources are available to check the safety of herbal medicines use in pregnancy, such as herbal medicines textbooks and websites[4,28,49].
Summary
With reference to different studies, the number of women who use herbal medicines during pregnancy has been increasing globally. This article outlined some of the main factors healthcare professionals need to consider when managing patients who choose to take herbal medicines.
- 1Traditional, complementary and integrative medicine. World Health Organization. https://www.who.int/health-topics/traditional-complementary-and-integrative-medicine#tab=tab_1 (accessed Sep 2022).
- 2Dippenaar J. Herbal and alternative medicine: the impact on anesthesia. Southern African Journal of Anaesthesia and Analgesia. 2015;21:8–13. doi:10.1080/22201181.2015.1013321
- 3Herbal medicines granted a traditional herbal registration. Medicines & Healthcare products Regulatory Agency. 2022.https://www.gov.uk/government/publications/herbal-medicines-granted-a-traditional-herbal-registration-thr/herbal-medicines-granted-a-traditional-herbal-registration (accessed Sep 2022).
- 4European Union herbal monographs. European Medicines Agency. https://www.ema.europa.eu/en/medicines/field_ema_herb_outcome/european-union-herbal-monograph-254/field_ema_web_categories%253Aname_field/Herbal (accessed Sep 2022).
- 5WHO global report on traditional and complementary medicine 2019. World Health Organisation. 2019.https://apps.who.int/iris/handle/10665/312342 (accessed Sep 2022).
- 6Laelago T. Herbal Medicine Use during Pregnancy: Benefits and Untoward Effects. In: Herbal Medicine. IntechOpen 2018. 314.https://www.intechopen.com/chapters/61138 (accessed Sep 2022).
- 7Illamola S, Amaeze O, Krepkova L, et al. Use of Herbal Medicine by Pregnant Women: What Physicians Need to Know. Front Pharmacol 2020;10:1483. doi:10.3389/fphar.2019.01483
- 8Muñoz B, Stewart D, Shetty A, et al. Herbal Medicinal Product Use During Pregnancy and the Postnatal Period: A Systematic Review. Obstet Gynecol 2019;133:920–32. doi:10.1097/AOG.0000000000003217
- 9Kennedy D, Lupattelli A, Koren G, et al. Herbal medicine use in pregnancy: results of a multinational study. BMC Complement Altern Med 2013;13:355. doi:10.1186/1472-6882-13-355
- 10Holst L, Nordeng H, Haavik S. Use of herbal drugs during early pregnancy in relation to maternal characteristics and pregnancy outcome. Pharmacoepidemiol Drug Saf 2008;17:151–9. doi:10.1002/pds.1527
- 11Adams J, Lui C, Sibbritt D, et al. Women’s use of complementary and alternative medicine during pregnancy: a critical review of the literature. Birth 2009;36:237–45. doi:10.1111/j.1523-536X.2009.00328.x
- 12Pallivalappila A, Stewart D, Shetty A, et al. Complementary and alternative medicine use during early pregnancy. Eur J Obstet Gynecol Reprod Biol 2014;181:251–5. doi:10.1016/j.ejogrb.2014.08.017
- 13Pallivalapila A, Stewart D, Shetty A, et al. Use of complementary and alternative medicines during the third trimester. Obstet Gynecol 2015;125:204–11. doi:10.1097/AOG.0000000000000596
- 14Forster D, Denning A, Wills G, et al. Herbal medicine use during pregnancy in a group of Australian women. BMC Pregnancy Childbirth 2006;6:21. doi:10.1186/1471-2393-6-21
- 15Westfall R. Herbal medicine in pregnancy and childbirth. Adv Ther 2001;18:47–55. doi:10.1007/BF02850250
- 16Hall H, McKenna L, Griffiths D. Midwives’ support for Complementary and Alternative Medicine: a literature review. Women Birth 2012;25:4–12. doi:10.1016/j.wombi.2010.12.005
- 17Nordeng H, Havnen G. Use of herbal drugs in pregnancy: a survey among 400 Norwegian women. Pharmacoepidemiol Drug Saf 2004;13:371–80. doi:10.1002/pds.945
- 18Mekuria A, Erku D, Gebresillassie B, et al. Prevalence and associated factors of herbal medicine use among pregnant women on antenatal care follow-up at University of Gondar referral and teaching hospital, Ethiopia: a cross-sectional study. BMC Complement Altern Med 2017;17:86. doi:10.1186/s12906-017-1608-4
- 19Laelago T, Yohannes T, Lemango F. Prevalence of herbal medicine use and associated factors among pregnant women attending antenatal care at public health facilities in Hossana Town, Southern Ethiopia: facility based cross sectional study. Arch Public Health 2016;74:7. doi:10.1186/s13690-016-0118-z
- 20Dugoua J. Herbal medicines and pregnancy. J Popul Ther Clin Pharmacol 2010;17:e370-8.https://www.ncbi.nlm.nih.gov/pubmed/21041871
- 21Ahmed M, Hwang J, Choi S, et al. Safety classification of herbal medicines used among pregnant women in Asian countries: a systematic review. BMC Complement Altern Med 2017;17:489. doi:10.1186/s12906-017-1995-6
- 22Bishop J, Northstone K, Green J, et al. The use of Complementary and Alternative Medicine in pregnancy: data from the Avon Longitudinal Study of Parents and Children (ALSPAC). Complement Ther Med 2011;19:303–10. doi:10.1016/j.ctim.2011.08.005
- 23Is it safe to take herbal medicines during pregnancy? Specialist Pharmacy Services. 2019.https://www.sps.nhs.uk/articles/is-it-safe-to-take-herbal-medicines-during-pregnancy/ (accessed Sep 2022).
- 24Kam P, Barnett D, Douglas I. Herbal medicines and pregnancy: A narrative review and anaesthetic considerations. Anaesth Intensive Care 2019;47:226–34. doi:10.1177/0310057X19845786
- 25Dugoua J, Seely D, Perri D, et al. Safety and efficacy of black cohosh (Cimicifuga racemosa) during pregnancy and lactation. Can J Clin Pharmacol 2006;13:e257-61.https://www.ncbi.nlm.nih.gov/pubmed/17085773
- 26Lead Poisoning in Pregnant Women Who Used Ayurvedic Medications from India — New York City, 2011–2012. Centers for Disease Control and Prevention. 2012.https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6133a1.htm (accessed Sep 2022).
- 27Jurgens TM. Potential toxicities of herbal therapies in the developing fetus. Dev Reproducers Toxic 2003;68:496–8.https://onlinelibrary.wiley.com/doi/abs/10.1002/bdrb.10050
- 28Natural Medicines database. Natural Medicines. www.naturalmedicines.com (accessed Sep 2022).
- 29Freyer AM. Drugs in Pregnancy and Lactation 8th Edition: A Reference Guide to Fetal and Neonatal Risk. Obstet Med. 2009;2:89–89. doi:10.1258/om.2009.090002
- 30Herbal Medicines. Medicines Complete. https://about.medicinescomplete.com/publication/herbal-medicines/ (accessed Oct 2022).
- 31Finkel RS, Zarlengo KM. Blue Cohosh and Perinatal Stroke. N Engl J Med. 2004;351:302–3. doi:10.1056/nejm200407153510323
- 32Reichert R. Neonatal Congestive Heart Failure Associated with Maternal Use of Blue Cohosh. J Pediatrics 1998;:550–2.http://www.encognitive.com/node/14645
- 33Dugoua J, Perri D, Seely D, et al. Safety and efficacy of blue cohosh (Caulophyllum thalictroides) during pregnancy and lactation. Can J Clin Pharmacol 2008;15:e66-73.https://www.ncbi.nlm.nih.gov/pubmed/18204101
- 34Kast RE. Borage oil reduction of rheumatoid arthritis activity may be mediated by increased cAMP that suppresses tumor necrosis factor-alpha. International Immunopharmacology. 2001;1:2197–9. doi:10.1016/s1567-5769(01)00146-1
- 35Toxbase. Toxbase. www.toxbase.org (accessed Oct 2022).
- 36Chuang C-H, Doyle P, Wang J-D, et al. Herbal Medicines Used During the First Trimester and Major Congenital Malformations. Drug Safety. 2006;29:537–48. doi:10.2165/00002018-200629060-00006
- 37Botanical Medicine for Women’s Health. Elsevier 2010. doi:10.1016/b978-0-443-07277-2.x0001-3
- 38Herbs and Pregnancy. American Pregnancy Association. https://americanpregnancy.org/healthy-pregnancy/is-it-safe/herbs-and-pregnancy/ (accessed Oct 2022).
- 39Traditional Herbal Medicines: a Guide to Their Safer Use. British Journal of Clinical Pharmacology. 2008;66:418–20. doi:10.1111/j.1365-2125.2008.03259.x
- 40The Management of Nausea andVomiting of Pregnancy and Hyperemesis Gravidarum. Green-top Guideline No. 69. Royal College of Obstetricians and Gynaecologists. . 2016.https://www.rcog.org.uk/media/y3fen1x1/gtg69-hyperemesis.pdf (accessed Oct 2022).
- 41Matthews A, Haas DM, O’Mathúna DP, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database of Systematic Reviews. 2015;2015. doi:10.1002/14651858.cd007575.pub4
- 42Antenatal care: routine care for the healthy pregnant woman. National Institute for Health and Care Excellence. 2019.http://www.nice.org.uk/CG62 (accessed Oct 2022).
- 43Fischer-Rasmussen W, Kjær SK, Dahl C, et al. Ginger treatment of hyperemesis gravidarum. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1991;38:19–24. doi:10.1016/0028-2243(91)90202-v
- 44Depression in adults: recognition and management. National Institute for Health and Care Excellence. 2009.https://www.nice.org.uk/guidance/cg90 (accessed Oct 2022).
- 45Dugoua J, Mills E, Perri D, et al. Safety and efficacy of St. John’s wort (hypericum) during pregnancy and lactation. Can J Clin Pharmacol 2006;13:e268-76.https://www.ncbi.nlm.nih.gov/pubmed/17085775
- 46Schäfer W, Wentzell N, Schink T, et al. Characterization of pregnancies exposed to St. John’s wort and their outcomes: A claims data analysis. Reproductive Toxicology. 2021;102:90–7. doi:10.1016/j.reprotox.2021.04.005
- 47Chan LY-S, Chiu P-Y, Lau T-K. A study of hypericin-induced teratogenicity during organogenesis using a whole rat embryo culture model. Fertility and Sterility. 2001;76:1073–4. doi:10.1016/s0015-0282(01)02730-3
- 48McLay J, Izzati N, Pallivalapila A, et al. Pregnancy, prescription medicines and the potential risk of herb-drug interactions: a cross-sectional survey. BMC Complement Altern Med 2017;17:543. doi:10.1186/s12906-017-2052-1
- 49Briggs G, Freeman R, Yaffe S. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 12th ed. Philadelphia, PA: : Lippincott–Williams and Wilkins 2021. https://about.medicinescomplete.com/publication/drugs-in-pregnancy-lactation/ (accessed Sep 2022).
- 50Royal College of Obstetricians and Gynaecologist Green-top Guidelines. Royal College of Obstetricians and Gynaecologists. https://www.rcog.org.uk/guidelines (accessed Sep 2022).
- 51El Shamy T, Tamizian O. Principles of prescribing in pregnancy. Obstet Gynaecol Reprod Med 2021;31:317–22. doi:https://doi.org/10.1016/j.ogrm.2021.09.004
- 52Russell P. The principles of prescribing in pregnancy. Specialist Pharmacy Service. 2021.https://www.sps.nhs.uk/articles/the-principles-of-prescribing-in-pregnancy/ (accessed Sep 2022).
- 53Bhamra S, Slater A, Howard C, et al. Health care professionals’ personal and professional views of herbal medicines in the United Kingdom. Phytother Res 2019;33:2360–8. doi:10.1002/ptr.6418
- 54Zahn R, Perry N, Perry E, et al. Use of herbal medicines: Pilot survey of UK users’ views. Complement Ther Med 2019;44:83–90. doi:10.1016/j.ctim.2019.02.007
- 55Skalli S, Zaid A, Soulaymani R. Drug interactions with herbal medicines. Ther Drug Monit 2007;29:679–86. doi:10.1097/FTD.0b013e31815c17f6
- 56Hardy M. Herbs of special interest to women. J Am Pharm Assoc (Wash) 2000;40:234–42; quiz 327–9. doi:10.1016/s1086-5802(16)31064-6
- 57The Yellow Card scheme: guidance for healthcare professionals, patients and the public. Medicines and Healthcare products Regulatory Agency. 2015.https://www.gov.uk/guidance/the-yellow-card-scheme-guidance-for-healthcare-professionals (accessed Sep 2022).
- 58UK Teratology Information Service . UK Teratology Information Service . http://www.uktis.org (accessed Sep 2022).
You might also be interested in…

Case-based learning: sedating medicines, breastfeeding and safe sleeping advice

Prenatal aspirin at earliest opportunity cuts severe pre-eclampsia risk, study finds
