Andor Bujdoso / Alamy Stock Photo
After reading this article, you should be able to:
- Explain the principles of prescribing in pregnancy and the applications in clinical practice;
- Understand the management of COVID-19 infection in pregnancy and the use of COVID-19 vaccines;
- Understand the evolving role of an obstetric clinical pharmacist as an advanced clinical practitioner.
The tragedy of thalidomide, a drug that was used to treat nausea and vomiting in pregnancy but caused severe limb deformities in babies in the 1950s and 1960s, led to the creation of the UK’s Committee on the Safety of Drugs (later, the Committee on the Safety of Medicines) and the Medicines Act of 1968[1,2]. Thalidomide was withdrawn from use in the UK in 1961, after being on the market for five years[1,2].
In addition to that legislation being passed, a range of supporting resources have been developed to improve the safety of drug use in pregnancy, including:
- Websites (e.g. TOXBASE);
- National guidelines (e.g. National Institute for Health and Care Excellence guidance and Royal College of Obstetricians and Gynaecologists ‘Green-top’ guidelines);
- Medication safety alerts relating to pregnancy from the Medicines and Healthcare products Regulatory Agency (MHRA);
- Academic resources that collate and synthesise findings from different clinical studies;
- Drug company datasheets that include information relevant to pregnancy;
- Specialised centres, such as the UK Teratology Information Service[3].
Despite this improved knowledge base, prescribing in pregnancy remains challenging for healthcare professionals. There are concerns about teratogenicity and how the physiological changes of pregnancy affect the pharmacokinetics and pharmacodynamics of medications[4]. A lack of robust clinical trials, owing, in part, to the ethical difficulties for recruiting pregnant women in studies, can result in limited information regarding potential adverse foetal effects and pregnancy outcomes of new medications[4,5].
In the UK, more than 80% of women report taking medicines during their pregnancy[5]. Recent studies in the UK estimated 65% of pregnant women had been prescribed at least one medication[4]. Congenital malformations are present in 2–3% of newborn babies, with approximately 1–2% of this total being associated with exposure to a teratogen[4,6].
The number of women needing to take medicines during pregnancy is increasing, partly because of advancing maternal age, increased incidence of pre-existing medical conditions that require pharmacotherapy (e.g. lupus, heart disease, transplant recipients), and/or the development of obstetric complications during pregnancy (e.g. gestational diabetes, obstetric cholestasis, COVID-19 infection)[4,6–9]. Hence, the provision of medical and pharmaceutical care in this group of patients becomes more complex.
It is important for the obstetric pharmacist to work closely with doctors, nurses, midwives and patients on care plans that optimise the safety and efficacy of drug use during pregnancy, and to provide patient counselling services when needed. It is also crucial for the specialised pharmacist to have a sound grasp of the literature and problem-solving skills when addressing queries on drug use in pregnancy.
Most of the drugs used in pregnancy are unlicensed in terms of the indications and the specific patient group because of a lack of robust clinical trials. However, the available national guidance, online evidence-based databases, relevant academic resources and professional advice available from specialised centres can all help healthcare professionals with decision-making on treatment options for pregnant women. There is also useful information available for patient counselling regarding drugs use in pregnancy[3].
Teratogenicity
Teratogenicity is the ability of a drug to cause foetal abnormalities or deformities[10]. Teratogens are defined as agents or factors that cross the placenta causing congenital malformations[6]. They can directly or indirectly cause structural or functional abnormalities in the foetus or in the infant after birth, some of which may not be apparent until later in the child’s life[5,6,10]. Examples of late-onset effects include adenocarcinoma of the vagina after puberty in females exposed to diethylstilbestrol in the womb, and adverse effects on intellectual, social and functional development[10].
Teratogens can cause harmful effects on the embryo or foetus at any time during pregnancy, but they do not always cause abnormalities in all foetuses that have been exposed[5]. For example, thalidomide caused abnormalities in less than half of all foetuses exposed during the critical period (for this drug, 20–36 days after fertilisation)[6].
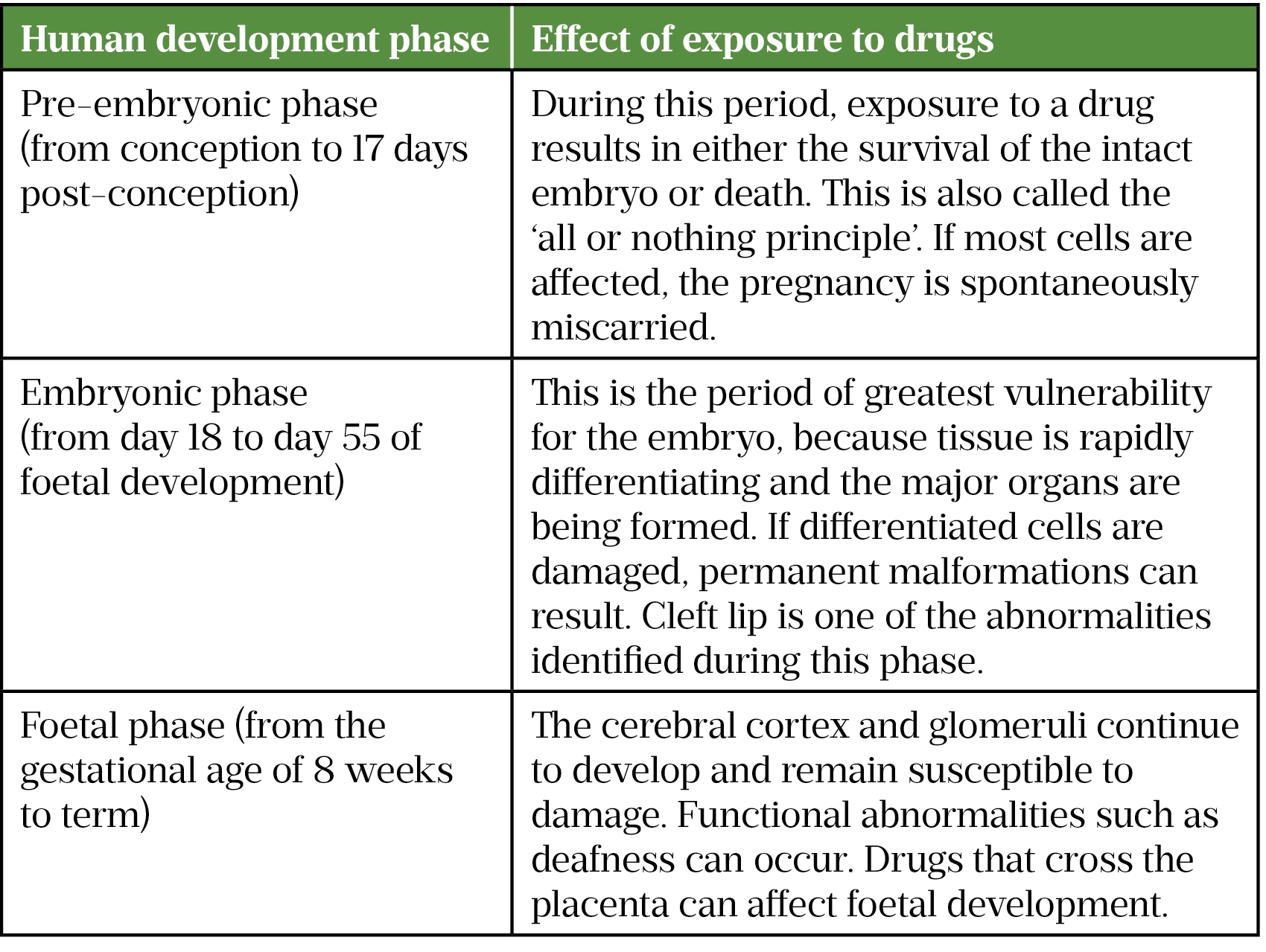
The foetal response to a teratogen is influenced by factors such as the dose, route and timing of exposure, and genetic and environmental factors[4,6,11]. An example of dose-dependent teratogenicity is the incidence of major congenital malformations with carbamazepine[6]. In addition, the risk of teratogenicity may also increase if the number of concomitant drugs is increased; for example, the incidence of foetal malformations increases with the number of antiepileptics taken[6]. The timing of exposure to a drug is a critical factor to determine the extent of any adverse effects and the type of birth defect. The three important phases in human development are illustrated in Table 1[4,6].
Most drugs cross the placenta to reach foetal circulation by simple diffusion[6]. The extent to which compounds will cross the placenta depends on their molecular size, degree of ionisation, protein binding and lipid solubility[6]. Non-ionised, lipid-soluble drugs will cross in preference to polar, ionised, hydrophilic compounds[6].
Drugs with higher molecular weight tend not to cross the placenta, but there are exceptions, such as infliximab and adalimumab[6]. Since adalimumab can cross the placenta into the serum of infants born to women treated with this drug, the manufacturer states that these infants may be at increased risk of infection. Therefore, administration of live vaccines to infants exposed to adalimumab in utero is not recommended for five months following the woman’s last adalimumab dose during pregnancy[12].
Table 2 lists drugs that are absolutely contraindicated in pregnancy[3,10,13–16].
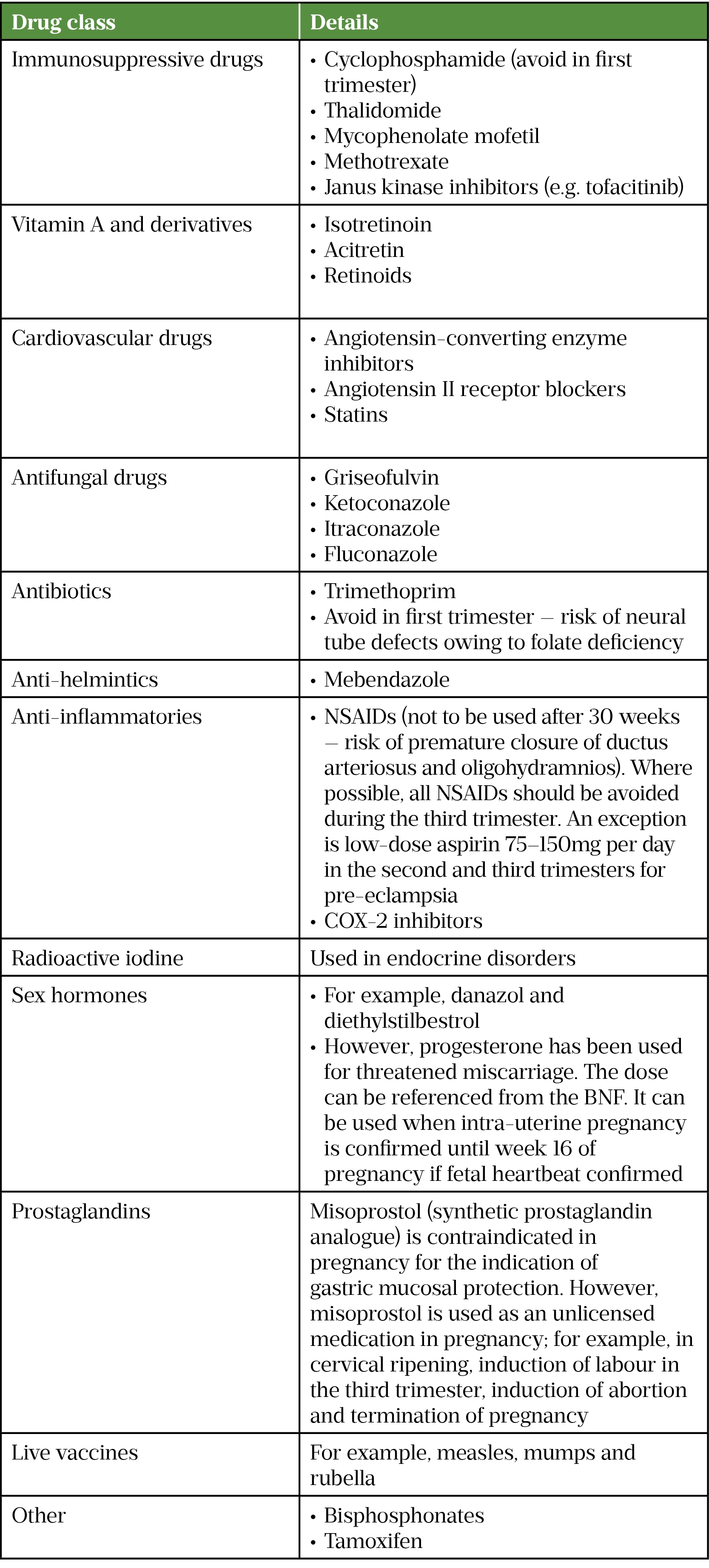
NSAIDs: non-steroidal anti-inflammatory drugs
Most drugs with a molecular weight of less than 1,500 Da can cross the placenta and potentially affect a foetus[16].
Drugs that are relatively contraindicated in pregnancy
Other drugs with proven teratogenic effects in humans that are advised to be avoided during pregnancy include:
- Lithium
- Antibiotics
- Tetracyclines (e.g. tetracycline, doxycycline, oxytetracycline). All tetracyclines are contraindicated in the second and third trimesters. These drugs can cause discolouration of deciduous teeth and may have transient effects on foetal bone growth. Tetracyclines may also exacerbate fatty liver of pregnancy. In animal studies, effects on skeletal development have been documented in the first trimester.
- Quinolones (e.g. ciprofloxacin, levofloxacin, moxifloxacin, nalidixic acid, norfloxacin and ofloxacin). Quinolones have been shown to cause arthropathy during neonatal exposure in animal studies.
- Chloramphenicol (oral and injection). Owing to the risk of serious haematological side effects, systemic use of chloramphenicol is reserved for the management of life-threatening infections. There are concerns that use near term may be associated with a risk of neonatal ‘grey baby syndrome’, in which the baby has a life-threatening reaction to chloramphenicol, one sign of which is an ashen grey colour of the skin; however, there are no well-documented cases of this occurring. The available data do not indicate that use of ocular chloramphenicol is associated with an increased incidence of congenital malformations; however, other outcomes have not been sufficiently studied to exclude a risk.
- Nitrofurantoin (avoid near term). There is a risk of causing haemolytic anaemia in newborns when used near term.
- Terbinafine (antifungal). Although the animal reproduction data are encouraging, the lack of human pregnancy experience does not allow a full assessment of the foetal risk from terbinafine. If possible, it would be safer to wait to start treatment until after a pregnancy has completed.
- Anticoagulants
- Warfarin
- Newer oral anticoagulants
- Dapsone (antileprotic; avoid in third trimester)
- Anti-malarials (avoid in first trimester)
- Mefloquine
- Primaquine
- Atovaquone with proguanil
- Artemether with lumefantrine
- Anticonvulsants
- Phenobarbitone
- Phenytoin
- Sodium valproate
- Valproate (anti-epileptic)
- Cardiovascular drugs
- Beta blockers (avoid the use of atenolol in first trimester)
- Minoxidil
- Diuretics (but appropriate to be used in treatment of pulmonary oedema)
- Spironolactone (feminisation has been observed in male rat foetuses)
- Endocrine drugs
- Octreotide
- Chlorpropamide[3,10,11,13,17–20].
If the benefit clearly outweighs the risk (e.g. in life-threatening disease), some ‘relatively contraindicated’ drugs can be used in pregnancy under specialist advice[13]. For example, warfarin is used in women with prosthetic heart valves disease, and anti-epileptic drugs are used in women with epilepsy[13]. The anti-epileptic drugs lamotrigine and levetiracetam are safer than other anti-epileptic drugs in pregnancy[20]. Such decisions should be agreed with the patient in a multidisciplinary team setting of senior clinical staff and after extensive patient counselling, if a less-teratogenic option is not available.
Role of the obstetric pharmacist
The role of the obstetric pharmacist has evolved from supplying medicines, screening in-patient drug charts and providing discharge prescriptions, to more advanced responsibilities[21,22]. Pharmacists address queries from patients, midwives and doctors in relation to drug use in pregnancy and breastfeeding, using the latest clinical evidence, and counsel patients when needed in the hospital and after discharge. Other roles include clinical guidelines review and patient group direction development, which involve: close liaison with the multidisciplinary team; evaluation of new drug applications; critical appraisal of unlicensed drugs use; antimicrobial stewardship; training and education for midwives, doctors and junior pharmacists; electronic prescribing enhancement; research and development, including pharmacogenetic areas, involvement of medication incidents management and suggestion of improvement action plans; and service development and quality improvement in a multidisciplinary team setting.
Box 1: Prescribing principles in pregnancy
Adherence to good, clear principles of prescribing together with patient involvement is essential when prescribing in pregnancy to ensure safe and effective use of medicines[5,6].
These principles are:
- Drugs should be prescribed in pregnancy only if the expected benefit (to the mother) is thought to be greater than the potential risk (to the foetus);
- Always carry out a risk–benefit assessment on an individual patient basis, and ensure that the most up-to-date evidence is used;
- Work in collaboration with the mother, ensuring that she is informed of any known risks or benefits of both taking and not taking the medication;
- If possible, all drugs should be avoided during the first trimester, which is the period of greatest susceptibility to teratogenic effects. The main risk is structural defects, because the major structures (e.g. brain, spinal cord, arms and legs) and organs are developing at this time;
- Only use a medicine when it is absolutely essential;
- Use the lowest effective dose for the shortest required duration;
- Consider non-pharmacological treatments (e.g. acupressure wrist bands for morning sickness);
- Drugs that have been extensively used in pregnancy and are usually safe should be prescribed in preference to new or untried drugs;
- Absence of information does not imply safety or lack of risk to the mother and the foetus;
- Avoid known human teratogens;
- When considering treatment for unfamiliar diseases, always seek advice from a specialist clinician;
- Avoid polypharmacy — teratogenicity of a medicine may be enhanced by co-administration of a second medicine or more;
- Offer pre-pregnancy counselling to all patients with chronic medical disorders. Many dedicated maternal medicine clinics offer this, particularly for complex disorders;
- Monitoring of any chronic medical condition should be intensified during pregnancy because the pattern of disease may change, as well as response to medicines;
- Provide adequate contraception to women on known teratogenics. For example, sodium valproate should be avoided in women with reproductive potential, but if no suitable alternative is available, effective contraception must also be offered to women not planning pregnancy;
- Accurate medicines reconciliation is crucial at transitions of care between wards or in the clinic. Herbal medicines and over-the-counter medicines should be included during drug history taking;
- Control of underlying diseases, such as arthritis, inflammatory bowel disease, epilepsy, asthma, and thyrotoxicosis with appropriate drug therapy is likely to reduce adverse and neonatal outcomes;
- Keep abreast of Medicines and Healthcare products Regulatory Agency alerts relating to the safety of drug use in pregnancy and apply the recommendations in practice[4–6,10,13,23].
Box 2: Practical advice for prescribing in pregnancy
- Some over-the-counter, herbal and vitamin products should be avoided in pregnancy because they may contain ingredients or quantities of ingredients that can cause harm to the foetus;
- Medicines given shortly before term or during labour can have adverse effects on labour or on the baby after delivery (e.g. withdrawal effects from opioids);
- Alcohol, tobacco and other recreational substances should be avoided during pregnancy. Offer counselling service, advice on nicotine replacement therapy or non-pharmaceutical therapy when appropriate;
- Drinking alcohol, especially in the first three months of pregnancy, increases the risk of miscarriage, premature birth and intrauterine growth restriction. Drinking after the first trimester affects the baby post-natally. Drinking heavily (more than six drinks per day) throughout pregnancy can cause the baby to develop foetal alcohol syndrome;
- Smoking during pregnancy can lead to increased spontaneous abortion, preterm birth, perinatal mortality, infants with low birth weight and stillbirths. Neonatal exposure is associated with sudden infant death syndrome, asthma, respiratory infections and attention deficit disorder;
- Consider maternal contraindications and precautions when advising on a drug in pregnancy, (e.g. avoid recommending labetalol for hypertension in an asthmatic patient);
- Closely monitor drugs that have a narrow therapeutic index during pregnancy;
- Vaccines made with a live virus (e.g. rubella and varicella vaccines) are not given to women who are, or may be, pregnant;
- For certain drugs requiring body weight for dose calculation (e.g. gentamicin injection or low-molecular-weight heparin), ensure that weight is measured at the most appropriate time. Some centres use the booking weight (at the 12th week of pregnancy) for dose calculation even though the drug is used during the second or third trimester. Always check the relevant local guidelines;
- All women should take folate supplements from the time pregnancy is planned and for the first 12 weeks of pregnancy to reduce the risks of neural tube defects (NTD) in the foetus. A higher daily dose (5mg daily) is recommended for women at a high risk of conceiving a child with NTD, including women who have previously had an infant with NTD, are receiving antiepileptic treatments, or have diabetes or sickle cell disease;
- In women diagnosed with hyperemesis gravidarum, a high dose of folic acid (5mg daily) is sometimes used, because vomiting affects the oral absorption of this drug;
- Venous thromboembolism risk increases with pregnancy because of changes in homeostasis and raised circulating blood volume. The risk is also increased in the presence of pre-existing risk factors, such as clotting disorders, obesity, a positive family history and smoking;
- Consider different routes of administration for drugs used to treat pregnancy complications, such as administering medicines by buccal or rectal route in conditions such as hyperemesis gravidarum;
- Information relating to drugs and pregnancy is available from the UK Teratology Information Service and the Best Use of Medicines in Pregnancy websites[3,5,6,10,13,17,23–28].
How pregnancy affects pharmacokinetics
Substantial anatomical and physiological changes occur during pregnancy. These changes can affect the pharmacokinetics (absorption, distribution, metabolism and excretion) of drugs in pregnant women[18,29,30]. Changes in pharmacokinetics during pregnancy can have an impact on drug efficacy and toxicity.
The overall changes in physiological parameters during pregnancy take place progressively throughout three trimesters[30,31]. The increase in cardiac output, total body water, fat compartment and glomerular filtration rate, as well as the decrease in plasma albumin concentration and altered activity of drug-metabolizing enzymes, are all reported to be at the highest level during the third trimester[30,31].
The major changes in pharmacokinetics during pregnancy are outlined in Table 3[18,30,32–37].

COVID-19 management in pregnancy
The majority of COVID-19-positive pregnant women are either asymptomatic or experience mild to moderate cold or flu-like symptoms[37]. Pregnant or recently pregnant women with COVID-19 are more than twice as likely to require admission into intensive care or require invasive ventilation, compared with non-pregnant women[38]. COVID-19 infection is also associated with an increased risk of pre-term birth and, although rare, stillbirth[37,39].
Maternal risk factors associated with both COVID-19 infection and admission to hospital include:
- Being unvaccinated;
- Age ≥35 years;
- Black or ethnic minority background;
- Body mass index ≥25kg/m2;
- Any pre-pregnancy comorbidity (e.g. chronic hypertension or diabetes)[37–39].
Recommendations for COVID-19 vaccination in pregnancy
COVID-19 vaccination is strongly advised for all pregnant women by the Royal College of Obstetricians and Gynaecologists[37,40]. Pregnant women should be offered the primary and reinforcing vaccination; the Pfizer-BioNTech or Moderna mRNA vaccines are the preferred options because of the large post-marketing experience[37,40]. Vaccination can be offered at any time during pregnancy[37]. If one dose of the Oxford/AstraZeneca vaccine is given, then the course should be completed either with a further dose of the same vaccine or an mRNA vaccine[37,40].
Patients should be advised of the benefits of vaccination during pregnancy, which include:
- Reduced risk of developing severe COVID-19 infection and requiring hospitalsation;
- Potential reduced risk of pre-term birth associated with COVID-19;
- Potential reduced risk of stillbirth associated with COVID-19;
- Potential protection for neonate via passive antibody transfer;
- Potential reduced risk of transmission to vulnerable household members[37].
Use of mRNA COVID-19 vaccines has been shown to produce similar antibody titres between pregnant and non-pregnant women and generate greater antibody titres, in comparison with active infection during pregnancy[41,42]. Vaccine-generated antibodies were found in umbilical cord blood and breast milk, indicating the passive transfer of antibodies from mother to neonate[37,41,42].
Although pregnant patients were not included in the large clinical trials investigating the safety profile of COVID-19 vaccines, current available data do not indicate any safety concerns associated with use in pregnancy[37,40,43,44]. The side effect profile of COVID-19 vaccines is similar between pregnant and non-pregnant women[41,44]. Pregnancy outcomes (specifically premature delivery, low birth weight and stillbirth) were similar in women vaccinated during pregnancy compared with pregnant women who were not vaccinated[44].
Research involving the safety, immunogenicity and scheduling of COVID-19 vaccines in pregnant women is ongoing[37]. This includes a worldwide, randomised, controlled trial in which pregnant women will receive either Pfizer-BioNTech vaccine or placebo to obtain safety and immunogenicity data (women given the placebo will be offered the vaccine once they give birth)[37,45]. The HORIZON 1 trial by Janssen plans to investigate the dosing schedule of the Ad26.COV2.S vaccine[37,45]. Lastly, the Preg-CoV trial, led by St George’s, University of London, aims to give pregnant women different vaccines on different schedules to identify the optimal vaccination schedule[37,46].
Management of COVID-19 in pregnancy
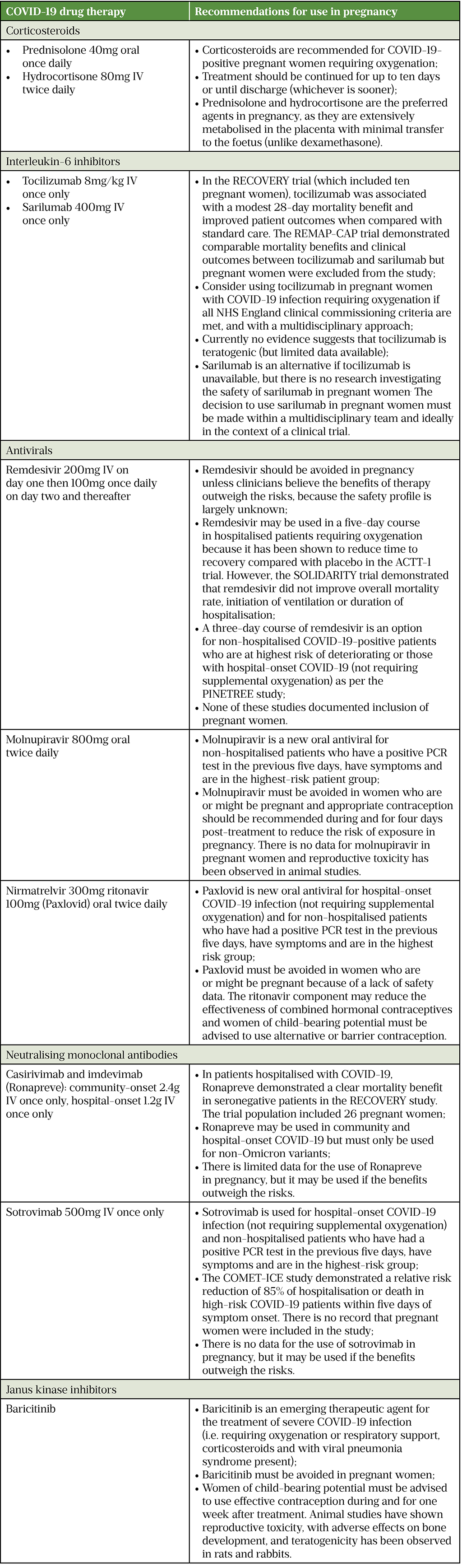
Pregnant women admitted to hospital with COVID-19 infection should be managed in the same manner as non-pregnant women: by using oxygen, venous thromboembolism prevention, corticosteroids, interleukin-6 inhibitors and monoclonal antibodies (where appropriate and if NHS England commissioning criteria are met)[37]. As the treatment of COVID-19 is constantly evolving, pharmacists must keep up to date with national recommendations from the Royal College of Obstetricians and Gynaecologists and the NHS. Table 4 outlines the current drug therapies available for COVID-19 and NHS England recommendations for use in pregnant women[21,37,38,47–62].
UK COVID-19 Antivirals Pregnancy Registry
As the safety of COVID-19 antivirals in pregnancy has not been established, healthcare staff, pregnant women and their partners are all advised to report use of antivirals taken around conception or during pregnancy (both maternal and paternal use) to the UK COVID-19 Antivirals Pregnancy Registry.
The UK COVID-19 Antivirals Pregnancy Registry is being operated by the MHRA in collaboration with the UK Teratology Information Service to collect information about exposures to COVID-19 antivirals in pregnancy and enable follow-up of any reported pregnancies; the registry is also collecting information on outcomes for pregnancies where conception occurred during or shortly after paternal exposure to antiviral treatment[63].
Summary
The antenatal and postnatal care of women is becoming increasingly complex, especially during the COVID-19 pandemic. The increased use of medications during pregnancy makes it a challenging area for healthcare professionals. The role of the obstetric clinical pharmacist has evolved from supply and prescription screening to that of a more advanced practitioner. The pharmacist is now required to be actively involved in patient care by collaborating closely with the multidisciplinary team and adopting an evidence-based approach. The specialised pharmacist also participates in guideline development, research and development, service improvement in the multidisciplinary team setting and critical evaluations of unlicensed drugs use. With these emerging clinical leadership roles, obstetric clinical pharmacy has become a rewarding and exciting career for pharmacists who have a special interest in this field and who enjoy working with a dedicated team of doctors, midwives, nurses and other healthcare staff.
- 1Vargesson N. Thalidomide‐induced teratogenesis: History and mechanisms. Birth Defect Res C. 2015;105:140–56. doi:10.1002/bdrc.21096
- 2Kim JH, Scialli AR. Thalidomide: The Tragedy of Birth Defects and the Effective Treatment of Disease. Toxicological Sciences. 2011;122:1–6. doi:10.1093/toxsci/kfr088
- 3Briggs G, Freeman R, Yaffe S. Drugs in Pregnancy & Lactation: A Reference Guide to Fetal and Neonatal Risk (12th edn). MedicinesComplete. 2022.https://about.medicinescomplete.com/publication/drugs-in-pregnancy-lactation/ (accessed Jul 2022).
- 4El Shamy T, Tamizian O. Principles of prescribing in pregnancy. Obstetrics, Gynaecology & Reproductive Medicine. 2021;31:317–22. doi:10.1016/j.ogrm.2021.09.004
- 5Russell P. The principles of prescribing in pregnancy. Specialist Pharmacy Service. 2021.https://www.sps.nhs.uk/articles/the-principles-of-prescribing-in-pregnancy/ (accessed Jul 2022).
- 6Pregnancy and medicines. Medicines Learning Portal. 2019.https://filestore.medicineslearningportal.org/docs/Pregnancy%20PDF%20v4.pdf (accessed Jul 2022).
- 7Ayad M, Costantine MM. Epidemiology of medications use in pregnancy. Seminars in Perinatology. 2015;39:508–11. doi:10.1053/j.semperi.2015.08.002
- 8Lupattelli A, Spigset O, Twigg MJ, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014;4:e004365. doi:10.1136/bmjopen-2013-004365
- 9Eléfant E, Hanin C, Cohen D. Pregnant women, prescription, and fetal risk. Handbook of Clinical Neurology. 2020;:377–89. doi:10.1016/b978-0-444-64150-2.00027-7
- 10British National Formulary. MedicinesComplete. 2022.https://about.medicinescomplete.com/publication/british-national-formulary/ (accessed Jul 2022).
- 11Gomes J do A, Olstad EW, Kowalski TW, et al. Genetic Susceptibility to Drug Teratogenicity: A Systematic Literature Review. Front. Genet. 2021;12. doi:10.3389/fgene.2021.645555
- 12Humira 40 mg/0.4 ml solution for injection in pre-filled pen. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/7986#PRODUCTINFO (accessed Jul 2022).
- 13Nelson-Piercy C. Appendix A.1: Prescribing in pregnancy. In: Handbook of Obstetric Medicines. Boca Raton, Florida: : CRC Press 2020. 344–346.
- 14Roberge S, Nicolaides K, Demers S, et al. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. American Journal of Obstetrics and Gynecology. 2017;216:110-120.e6. doi:10.1016/j.ajog.2016.09.076
- 15Tsamantioti E, Hashmi MF. Teratogenic Medications. StatPearls. 2021.https://www.ncbi.nlm.nih.gov/books/NBK553086/ (accessed Jul 2022).
- 16Chatsis V, Frey N. Misoprostol for Cervical Ripening and Induction of Labour: A review of Clinical Effectiveness, CostEffectiveness and Guidelines. CADTH. 2018.https://www.ncbi.nlm.nih.gov/books/NBK538944/pdf/Bookshelf_NBK538944.pdf (accessed Jul 2022).
- 17Reducing the Risk of Thrombosis and Embolism during Pregnancy and the Puerperium (Green-top Guideline No. 37a). Royal College of Obstetricians and Gynaecologists. 2015.www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf (accessed Jul 2022).
- 18Pinheiro EA, Stika CS. Drugs in pregnancy: Pharmacologic and physiologic changes that affect clinical care. Seminars in Perinatology. 2020;44:151221. doi:10.1016/j.semperi.2020.151221
- 19Safety review of epilepsy medicines in pregnancy – women who may become pregnant urged to discuss treatment options with their doctor. Medicines and Healthcare products Regulatory Agency. 2021.https://www.gov.uk/government/news/safety-review-of-epilepsy-medicines-in-pregnancy-women-who-may-become-pregnant-urged-to-discuss-treatment-options-with-their-doctor (accessed Jul 2022).
- 20Spironolactone Tablets 100mg. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/5919/smpc#PREGNANCY (accessed Jul 2022).
- 21Gnadt S. Clinical pharmacy and obstetrics. American Journal of Health-System Pharmacy. 2020;77:1941–4. doi:10.1093/ajhp/zxaa289
- 22Briggs GG. Pharmacists in obstetrics. American Journal of Health-System Pharmacy. 2018;75:92–92. doi:10.2146/ajhp170662
- 23The Management of Nausea and Vomiting of Pregnancy and Hyperemesis Gravidarum (Green-top Guideline No.69). Royal College of Obstetricians and Gynaecologists. 2016.www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg69-hyperemesis.pdf (accessed Jul 2022).
- 24Brown B, Wright C. Safety and efficacy of supplements in pregnancy. Nutrition Reviews. 2020;78:813–26. doi:10.1093/nutrit/nuz101
- 25Drinking alcohol while pregnant. NHS. 2020.https://www.nhs.uk/pregnancy/keeping-well/drinking-alcohol-while-pregnant/ (accessed Jul 2022).
- 26Stop smoking in pregnancy. NHS. 2019.https://www.nhs.uk/pregnancy/keeping-well/stop-smoking/ (accessed Jul 2022).
- 27Management of Sickle Cell Disease in Pregnancy. Royal College of Obstetricians and Gynaecologists. 2011.https://www.rcog.org.uk/media/nyinaztx/gtg_61.pdf (accessed Jul 2022).
- 28Lussana F, Coppens M, Cattaneo M, et al. Pregnancy-related venous thromboembolism: Risk and the effect of thromboprophylaxis. Thrombosis Research. 2012;129:673–80. doi:10.1016/j.thromres.2012.01.017
- 29Tasnif Y, Morado J, Hebert M. Pregnancy-related pharmacokinetic changes. Clin Pharmacol Ther 2016;100:53–62. doi:10.1002/cpt.382
- 30Pariente G, Leibson T, Carls A, et al. Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review. PLoS Med 2016;13:e1002160. doi:10.1371/journal.pmed.1002160
- 31Costantine M. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 2014;5:65. doi:10.3389/fphar.2014.00065
- 32Anderson GD. Pregnancy-Induced Changes in Pharmacokinetics. Clinical Pharmacokinetics. 2005;44:989–1008. doi:10.2165/00003088-200544100-00001
- 33Anderson G. Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert Opin Drug Metab Toxicol 2006;2:947–60. doi:10.1517/17425255.2.6.947
- 34Koren G, Pariente G. Pregnancy- Associated Changes in Pharmacokinetics and their Clinical Implications. Pharm Res. 2018;35. doi:10.1007/s11095-018-2352-2
- 35Chan M, Mainland P, Gin T. Minimum alveolar concentration of halothane and enflurane are decreased in early pregnancy. Anesthesiology 1996;85:782–6. doi:10.1097/00000542-199610000-00013
- 36Palahniuk R, Shnider S, Eger E. Pregnancy decreases the requirement for inhaled anesthetic agents. Anesthesiology 1974;41:82–3. doi:10.1097/00000542-197407000-00021
- 37Coronavirus (COVID-19), infection in pregnancy. Royal College of Obstetricians and Gynaecologists. 2022.https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/coronavirus-covid-19-infection-in-pregnancy (accessed Jul 2022).
- 38Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. doi:10.1136/bmj.m3320
- 39Vousden N, Bunch K, Morris E, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: A national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS One 2021;16:e0251123. doi:10.1371/journal.pone.0251123
- 40COVID-19: the green book, chapter 14a. UK Health Security Agency. 2022.www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a (accessed Jul 2022).
- 41Shimabukuro T, Kim S, Myers T, et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N Engl J Med 2021;384:2273–82. doi:10.1056/NEJMoa2104983
- 42Gray K, Bordt E, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol 2021;225:303.e1-303.e17. doi:10.1016/j.ajog.2021.03.023
- 43Kroger A, Plotkin S. Vaccines (General Immunisation Practices) . 6th ed. Philadelphia: : Saunders 2012.
- 44COVID-19 vaccine surveillance report: Week 14. UK Health Security Agency. 2022.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1067158/vaccine-surveillance-report-week-14.pdf (accessed Jul 2022).
- 45Study to Evaluate the Safety, Tolerability, and Immunogenicity of SARS CoV-2 RNA Vaccine Candidate (BNT162b2) Against COVID-19 in Healthy Pregnant Women 18 Years of Age and Older. ClinicalTrials.gov. 2022.https://clinicaltrials.gov/ct2/show/NCT04754594 (accessed Jul 2022).
- 46A Study of Ad26.COV2.S in Healthy Pregnant Participants (COVID-19) (HORIZON 1) . ClinicalTrials.gov. 2022.https://clinicaltrials.gov/ct2/show/NCT04765384 (accessed Jul 2022).
- 47Saad A, Chappell L, Saade G, et al. Corticosteroids in the Management of Pregnant Patients With Coronavirus Disease (COVID-19). Obstet Gynecol 2020;136:823–6. doi:10.1097/AOG.0000000000004103
- 48RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637–45. doi:10.1016/S0140-6736(21)00676-0
- 49Brown M, Alazawi W, Kanoni S. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med 2021;385:1147. doi:10.1056/NEJMc2108482
- 50Interim Clinical Commissioning Policy: IL-6 inhibitors (tocilizumab or sarilumab) for hospitalised patients with COVID-19 (adults). NHS England. 2022.www.england.nhs.uk/coronavirus/documents/interim-clinical-commissioning-policy-il-6-inhibitors-tocilizumab-or-sarilumab-for-hospitalised-patients-with-covid-19-adults-2/#position (accessed Jul 2022).
- 51Interim Clinical Commissioning Policy: Remdesivir for patients hospitalised due to COVID-19 (adults and adolescents 12 years and older). NHS England. 2022.https://www.england.nhs.uk/coronavirus/documents/interim-clinical-commissioning-policy-remdesivir-for-patients-hospitalised-due-to-covid-19-adults-and-adolescents-12-years-and-older/ (accessed Jul 2022).
- 52Beigel J, Tomashek K, Dodd L, et al. Remdesivir for the Treatment of Covid-19 – Final Report. N Engl J Med 2020;383:1813–26. doi:10.1056/NEJMoa2007764
- 53WHO Solidarity Trial Consortium., Pan H, Peto R, et al. Repurposed Antiviral Drugs for Covid-19 – Interim WHO Solidarity Trial Results. N Engl J Med 2021;384:497–511. doi:10.1056/NEJMoa2023184
- 54Gottlieb R, Vaca C, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med 2022;386:305–15. doi:10.1056/NEJMoa2116846
- 55Interim Clinical Commissioning Policy: Antivirals or Neutralising Monoclonal Antibodies for Non-Hospitalised Patients with COVID-19 (Version 5). NHS. 2022.https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=103968 (accessed Jul 2022).
- 56Interim Clinical Commissioning Policy: Antivirals or neutralising monoclonal antibodies in the treatment of hospital-onset COVID-19. NHS England. 2022.https://www.england.nhs.uk/coronavirus/publication/interim-clinical-commissioning-policy-antivirals-or-neutralising-monoclonal-antibodies-in-the-treatment-of-hospital-onset-covid-19/ (accessed Jul 2022).
- 57Paxlovid 150 mg/100 mg film-coated tablets. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/13145/smpc#PREGNANCY (accessed Jul 2022).
- 58RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2022;399:665–76. doi:10.1016/S0140-6736(22)00163-5
- 59COVID-19 therapeutic alert: neutralising monoclonal antibodies in the treatment of COVID-19 in hospitalised patients. Central Alerting System. 2021.https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103186 (accessed Jul 2022).
- 60Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med 2021;385:1941–50. doi:10.1056/NEJMoa2107934
- 61COVID-19 Therapeutic Alert: Baricitinib for Patients Hospitalised Due to COVID-19 (Adults and Children Aged 2 Years and Over). Central Alerting System. 2022.https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103203 (accessed Jul 2022).
- 62Olumiant 2 mg Film-Coated Tablets. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/2434/smpc#PREGNANCY (accessed Jul 2022).
- 63COVID-19 antivirals: reporting to the UK COVID-19 Antivirals Pregnancy Registry. Medicines and Healthcare products Regulatory Agency. 2022.https://www.gov.uk/drug-safety-update/covid-19-antivirals-reporting-to-the-uk-covid-19-antivirals-pregnancy-registry (accessed Jul 2022).