Sovereign / ISM / Science Photo Library
Summary box
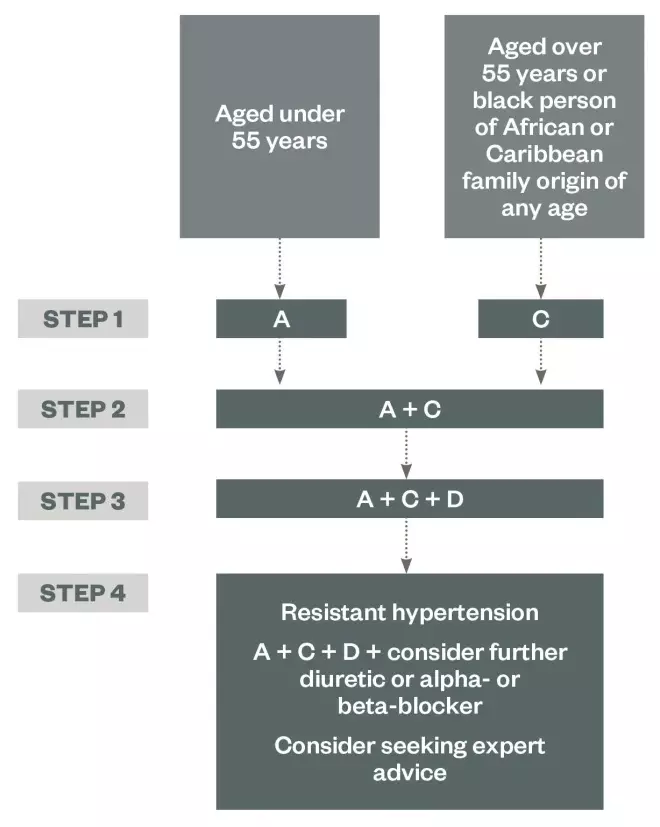
All patients with hypertension should be encouraged to make lifestyle changes, including weight loss, exercise and a reduced-salt diet. Patients with a blood pressure of 160/100 mmHg, or those with conditions such as diabetes or renal disease, should start pharmacological treatment. This depends on the patient’s age and ethnicity. Patients aged under 55 years of age are typically given an ACE inhibitor, while those older than 55 years or of black African or Caribbean ethnicity take a calcium-channel blocker.
Patients take a second medicine at step two of treatment, and a third (typically a thiazide-like diuretic) at step three. Patients with resistant hypertension, which is estimated to affect 10 million patients worldwide, may also take a further diuretic, or an alpha-blocker or beta-blocker.
The management of hypertension relies primarily on lifestyle modification with the addition of antihypertensive drug therapy in selected patients.
Lifestyle change is the treatment of choice for patients with stage 1 hypertension (blood pressure ≥140/90 mmHg but <160/100mmHg)
and a low or moderate calculated cardiovascular risk (<20% over 10 years)
[1]
.
However, all patients with hypertension at any stage should be offered lifestyle advice tailored to their individual cardiovascular risk factors.
Lifestyle changes have been shown to have a substantial beneficial effect on blood pressure (see ‘Lifestyle changes and blood pressure’), as well as on other cardiovascular risk factors, such as lipids and levels of obesity.
| Lifestyle changes and blood pressure[2] | ||
|---|---|---|
Modification | Recommendation | Approximate reduction in systolic |
Weight loss | Maintain normal body weight | 5–20 mmHg per 10 kg weight loss |
DASH-type diet (dietary approaches to stop hypertension) | Consume a diet rich in fruits, vegetables and low-fat dairy products with reduced saturated and total fat | 8–14 mmHg |
Reduced salt intake | Reduce daily dietary sodium intake as much as possible | 2–8 mmHg |
Physical activity | Undertake regular aerobic physical activity (at least 30 minutes per day, most days of the week) | 4–9 mmHg |
Moderation of alcohol intake | Limit consumption to two drinks per day in men and one drink per day in women and those of light weight | 2–4 mmHg |
Treatment guidelines for hypertension vary by geographical area. Guidelines from England’s National Institute for Health and Care Excellence (NICE) and the European Society of Cardiology are similar, whereas guidelines from the American Heart Association and the American College of Cardiology recommend more aggressive treatment.
The NICE guidelines recommend that pharmacological treatment is started in all patients with stage 2 hypertension (blood pressure ≥160/100 mmHg) or greater. It should also be started in patients with stage 1 hypertension in patients with evidence of target organ damage, diabetes, renal disease, or a high calculated cardiovascular risk (≥20% over 10 years)[1]
. Target organ damage includes damage to the heart, such as left ventricular hypertrophy, angina or prior myocardial infarction and heart failure; to the brain, such as stroke or transient ischaemic attack; peripheral arterial disease; or retinopathy.
The choice of treatment depends on the age and ethnicity of the patient (see ‘Summary of antihypertensive drug treatment’). In August 2011, NICE made significant changes to its treatment algorithm, including:
- recommending the use of a calcium-channel blocker in preference to a thiazide or related diuretic at step one
- the use of an angiotensin-II receptor antagonist (also known as an angiotensin receptor blocker, ARB) in preference to an angiotensin-converting enzyme (ACE) inhibitor at step two in people of African or Caribbean ethnicity
- the use of indapamide or chlortalidone in preference to bendroflumethiazide when introducing a thiazide or related diuretic at step three
- the use of additional diuretic therapy, including spironolactone, as a preferred option at step four.
Choice of treatment for hypertension[1]
Summary of antihypertensive drug treatment
How to choose an appropriate treatment for patients with hypertension.
Key:
A – ACE inhibitor or low-cost ARB
C – Calcium-channel blocker
D – Thiazide-like diuretic
Source: NICE
Treatment targets
The aim of treatment in simple hypertension for patients younger than 80 years of age should be to achieve a blood pressure of ≤140/90 mmHg, as there is insufficient evidence to support lower blood pressure targets
[3]
. If ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) is used to monitor response to treatment, the aim is to achieve an average daytime blood pressure of <135/85 mmHg. Patients with co-morbidities, such as chronic kidney disease (CKD), may require more aggressive blood pressure lowering.
The aim of treatment in patients aged older than 80 years of age who require pharmacological treatment is to achieve a blood pressure target of <150/90 mmHg (average daytime blood pressure <145/85mmHg if ABPM or HBPM is used), as more aggressive treatment to achieve lower blood pressure targets may be associated with poorer outcomes. The treatment threshold for patients aged older than 80 years is identical to that for younger patients[1]
.
Angiotensin-converting enzyme inhibitors
A key driver of hypertension is the production of angiotensin II, a potent vasoconstrictor, as a result of increased renin levels in the kidney. ACE inhibitors lower levels of angiotensin II by preventing the conversion of angiotensin I to angiotensin II mediated by angiotensin-converting enzyme. ACE inhibitors also reduce circulating aldosterone, which is responsible for sodium and water retention.
The use of ACE inhibitors is recommended as a first-line option for patients aged less than 55 years of age (except those of black African and Caribbean origin). They should also be used first-line in people with diabetes (owing to the additional benefit of renal protection), and are a suitable option for people with heart failure and prior myocardial infarction.
Patients of black African and Caribbean origin are at increased risk of angioedema with ACE inhibitors. The mechanism for this is unclear, but it may be due to an increased sensitivity to bradykinin[4]
.
First-dose hypotension can occur when an ACE inhibitor is first used, but this is unusual in hypertensive patients. The risk is reduced by using longer-acting medicines (such as ramipril, perindopril and trandolapril) and by reducing the dose of any diuretic the patient is taking.
The use of ACE inhibitors is associated with a risk of hyperkalaemia, in which the blood potassium level is raised, so the patient’s serum potassium level should be measured when starting an ACE inhibitor, then periodically thereafter (see ‘Monitoring medicines used in hypertension’). Patients should stop taking any potassium-sparing diuretics or potassium supplements before an ACE inhibitor is started, and care should be taken when using an ACE inhibitor with other drugs that may cause hyperkalaemia (such as spironolactone).
Serum creatinine and estimated glomerular filtration rate (eGFR) should be checked before ACE inhibitors are started in patients with impaired renal function, within two weeks of starting ACE inhibitors, and after any dose increases. A small change in eGFR (up to 20%) is to be expected on initiation or after dose increases, but if the patient experiences larger changes, stopping the ACE inhibitor and using an alternative treatment should be considered.
In patients with confirmed CKD, NICE recommends that treatment with ACE inhibitors (or ARBs) should be stopped if serum potassium concentrations increase to 6.0 mmol/l or more and other medicines known to promote hyperkalaemia have been discontinued[5]
.
The use of ACE inhibitors should be avoided in patients with known bilateral renal artery stenosis.
The most common side effect of long-term treatment with ACE inhibitors is a dry, irritating cough. This can occur several months after starting therapy and is thought to be due to an excess of bradykinin. Coughs caused by ACE inhibitors do not respond to cough medicines and may become troublesome for patients. If this is the case, an ARB can be used as an alternative.
Angiotensin-II receptor antagonists
ARBs interrupt the renin–angiotensin–aldosterone system by blocking the angiotensin-II receptor site. They have little effect on bradykinin, so they are less likely to cause a dry cough than ACE inhibitors.
In practice, NICE recommends that ARBs can be used as an alternative to ACE inhibitors, particularly in patients with a dry cough from using an ACE inhibitor. Note that ARBs should also be used in preference to ACE inhibitors in patients of black African or Caribbean ethnicity (if required at step two of treatment) as this particular group has an increased risk of angioedema with ACE inhibitors. ARBs should be monitored in the same way as ACE inhibitors.
Calcium-channel blockers
Calcium-channel blockers block the entry of calcium through the slow channels in cardiac and smooth muscle. This affects both muscle contraction and the conduction of cardiac impulses.
The effect of calcium-channel blockers is dependent on the affinity of the particular medicine for cardiac or smooth muscle.
For hypertension, calcium-channel blockers are recommended as the first-line treatment of choice in patients older than 55 years of age, and in people of black African or Caribbean ethnicity. The dihydropyridine calcium-channel blockers (such as amlodipine, nifedipine and felodipine) are most frequently prescribed because these have a greater impact on the vascular smooth muscle, which reduces peripheral resistance, and less effect on the myocardium. Verapamil or diltiazem are also sometimes used in patients with hypertension and concurrent supraventricular tachycardias, as these have an additional rate-controlling effect because of their action on cardiac muscle.
Calcium-channel blockers are generally well tolerated. Headache and facial flushing can occur for a few days after starting treatment but is usually self-limiting. The most common long-term effect is ankle swelling, which many patients accept but some find unsightly.
Shorter-acting formulations of the dihydropyridines have been associated with increased risk of cardiovascular events, so long-acting formulations are recommended[6]
.
Diuretics
Diuretics reduce sodium and water retention by inhibiting sodium reabsorption in the nephron of the kidney (see ‘Diuretic therapy explained’, online at www.pharmaceutical-journal.com). It is thought that the resulting diuresis causes an initial decrease in circulating volume and hence a reduction in cardiac output. This decreases the patient’s blood pressure and peripheral flow, which leads to a decrease in peripheral resistance as a result of autoregulation. Overall, the cardiac output is maintained with a net reduction in blood pressure. Thiazides and related diuretics also have a direct relaxant effect on vascular smooth muscle.
The NICE guidelines recommend that thiazide and related diuretics are added at step three of treatment, although they can be considered as an alternative to a calcium-channel blocker at step one in patients with evidence of heart failure or peripheral oedema (usually elderly patients). NICE recommends the use of indapamide or chlortalidone in preference to other thiazide and related diuretics, as these agents have been shown to reduce cardiovascular events when prescribed for the management of hypertension at low dose[1]
.
Thiazide and related diuretics are associated with significant hypokalaemia, and the patient’s urea and electrolytes should be measured four weeks after starting therapy and then periodically. Patients should also be monitored for dehydration resulting from fluid loss.
Thiazide and related diuretics should be avoided in patients with diabetes, a history of gout, or severe renal and liver impairment; with the exception of metolazone, this class becomes ineffective in patients with a glomerular filtration rate of <20 ml/min.
Loop diuretics are less effective as antihypertensives than thiazide and related diuretics (possibly owing to a reduced effect on vascular smooth muscle), and are reserved for use in those requiring more potent diuresis, for example in heart failure or where there is significant renal impairment.
| Monitoring medicines used in hypertension | |
|---|---|
Class | Parameters |
|
|
|
|
|
|
Resistant hypertension
Resistant hypertension is defined as blood pressure that remains higher than 140/90 mmHg after treatment with the optimal or maximum tolerated dose of an ACE inhibitor or ARB, a calcium-channel blocker, and a diuretic.
Resistant hypertension is thought to affect around 500,000 people in the UK[7]
, and around 10 million people worldwide[8]
. This is likely to increase as we see a greater proportion of older patients with co-morbidities.
There are few data from clinical studies on which to base decisions at step four of treatment. NICE recommends that low-dose spironolactone (25 mg once daily) should be considered in patients with a serum potassium level of 4.5 mmol/l or lower. Patients taking spironolactone with a reduced eGFR (<60 ml/min/1.73m2) are at increased risk of hyperkalaemia.
A higher dose of a thiazide or related diuretic should be considered in patients with a serum potassium level higher than 4.5 mmol/l. Other options at this step are an alpha-blocker (such as doxazosin) or a beta-blocker (such as bisoprolol). Patients should be referred to a specialist if the blood pressure remains uncontrolled with the patient taking the optimal or maximum tolerated doses of four drugs for hypertension.
Adherence
Hypertension is asymptomatic in most patients, and willingness to start and persist with therapy can be poor, especially if the medicines cause adverse effects. It is therefore essential that patients are fully aware of the consequences of persistent high blood pressure, including the increased risk of myocardial infarction, stroke and renal dysfunction. Patients should be encouraged to be actively involved in decisions about their care, and to discuss any concerns they may have.
The following should be considered:
- Identify and address any beliefs and concerns the patient has about their medicines.
- Use interventions to overcome practical problems if there is a specific need. Interventions might include: suggesting that patients record their medicine-taking; encouraging patients to monitor their condition; simplifying the dose regimen; using alternative packaging; or using a multi-compartment medicines system.
- Identify whether side effects are a problem and discuss the benefits, side effects and long-term effects and how the patient would like to deal with side effects. Consider adjusting the dose, switching to another medicine, and adopting other strategies, such as changing the timing of medicines.
Renal denervation
Renal denervation is an experimental intervention used to interrupt the neurogenical reflexes implicated in hypertension by radiofrequency denervation of the renal artery. Initial trial data show positive effects on blood pressure in the short and medium term from unblinded clinical studies. However, recent data from a large randomised blinded clinical trial failed to show a significant beneficial effect on blood pressure. As a result, the British Hypertension Society recommends that this technique should currently only be undertaken in clinical trials until a robust evidence base is established[9]
.
Helen Williams is a consultant pharmacist for cardiovascular disease at Southwark Clinical Commissioning Group.
References
[1] National Institute for Health and Care Excellence. Clinical Management of Primary Hypertension in Adults. London: NICE, 2011.
[2] Williams B, Poulter NR, Brown MJ et al. Guidelines for Management of Hypertension: Report of the Fourth Working Party of the British Hypertension Society, 2004 — BHS IV. J. Hum. Hypertension 2004, 18: 139–185.
[3] Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2013 ESH/ESC Guidelines for the management of arterial hypertension. J. Hypertens. 2013 31(7):1281–1357.
[4] Gibbs CR, Lip GYH & Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br. J. Clin. Pharmacol. 1999, 48(6): 861–865.
[5] National Institute for Health and Care Excellence. Chronic Kidney Disease: Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care. London: NICE, 2014.
[6] Opie L & Schall R. Evidence-based evaluation of calcium channel blockers for hypertension. J. Am. Coll. Cardiol. 2002, 39(2): 315–322.
[7] Caulfield M, de Belder M, Cleveland T et al. The Joint UK Societies’ Consensus Statement on Renal Denervation for Resistant Hypertension. London: BHSOC, 2012.
[8] Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011, 57: 1076–1080.
[9] The Joint UK Societies Working Group on Renal Denervation. Initial Response to the Medtronic Symplicity HTN3 Announcement. London: BHSOC, 2014.