James Cavallini/SCIENCE PHOTO LIBRARY
After reading this article, you should be able to:
- Define infective endocarditis (IE) and its pathophysiology;
- Identify patients that are at an increased risk of developing IE;
- Understand the most common pathogens responsible for IE;
- Understand the diagnosis and treatment options for IE.
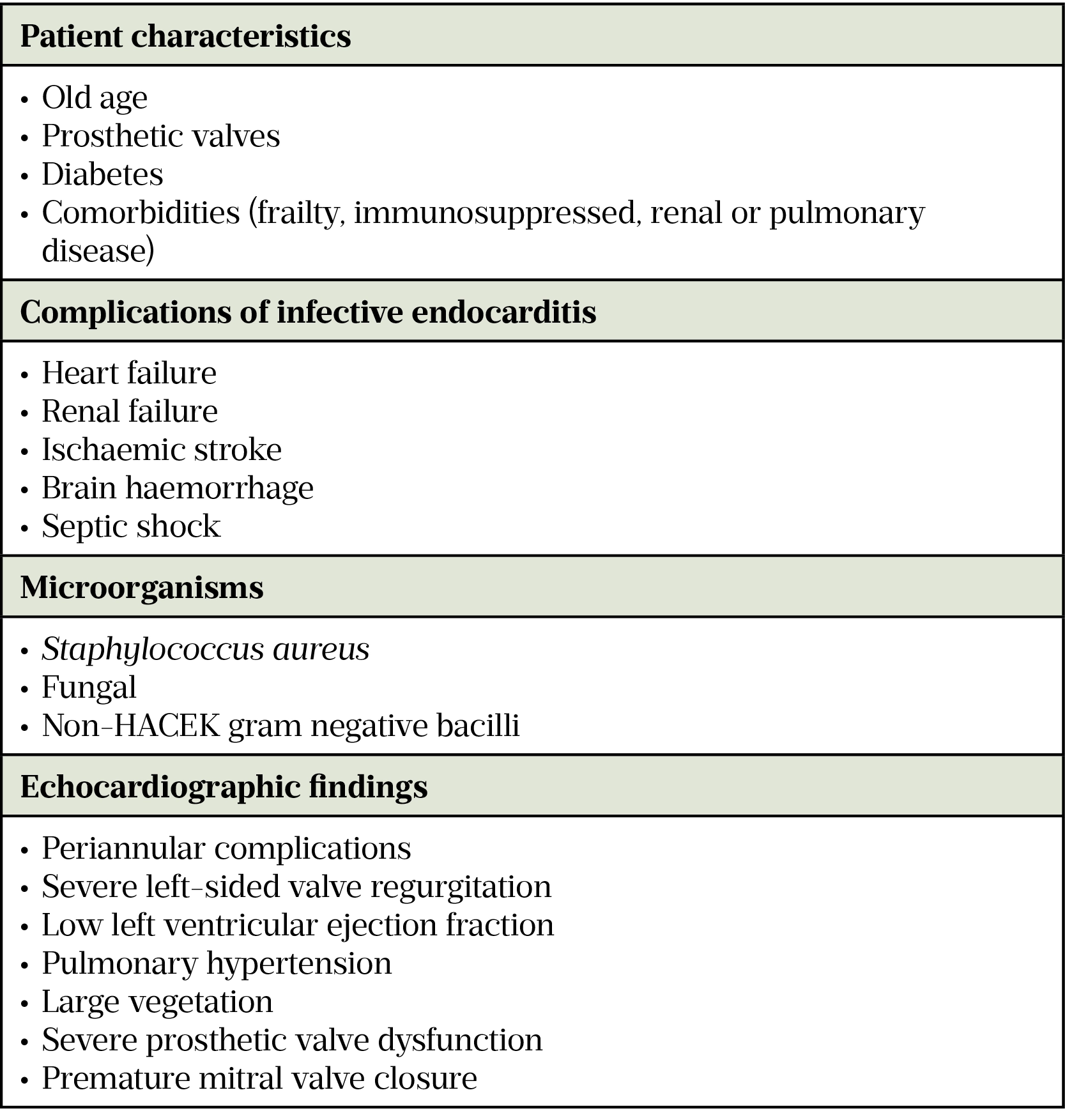
Infective endocarditis (IE) is defined as an infection of the endocardial surface, the cardiac valves (native or prosthetic) or an indwelling cardiac device[1]. IE is associated with high morbidity and mortality; in-hospital mortality rates range from 15–30% while the five-year mortality rate can reach 40%[1–4]. Patient outcomes are worse in the presence of certain factors (see Table 1)[5]. Approximately 40–50% of infected patients require valve surgery[2,6].
In low-income countries, IE is frequently seen in young adults with rheumatoid fever, which is precipitated by recent oral streptococcal infection[7]. Cases are generally lower in high-income countries (around 3–9 cases per 100,000 population each year) owing to high antibacterial usage; however, increasing antimicrobial resistance and an ageing population has made management more complex[2]. Incidence of IE within the UK appears to be on the rise, with completed consultant episodes increasing from 3,969 to 10,097 from 2009/2010 to 2019/2020[8,9]. The rise in cases of IE is likely to be multifactorial, owing to patient longevity, an increasing number of implantable cardiac devices, prosthetic valves and indwelling catheters, improved diagnostic modalities and more frequent hospital-acquired infections[10]. High-risk activities, such as intravenous drug usage, increase the risk of IE. Non-sterile injection practice can introduce opportunistic bacterial infection and impurities in the drugs can cause endocardial tissue damage[11].
This article will provide an overview of the pathogenesis of IE, as well as the assessment and diagnosis of the condition. It will also outline the management and role of the pharmacist.
Pathogenesis of infective endocarditis
The pathogenesis of IE is through the sequential stages of endothelial damage, adhesion of a bloodstream-borne pathogen and proliferation of the pathogen to cause local infection[12]. Normal endothelial lining of the heart and valves is sterile and naturally resistant to colonisation by microorganisms, but following endothelial damage or injury, platelets and fibrin may form a thrombus with inflammatory cells to which microorganisms can adhere, forming an infected vegetation (irregular growths formed of microorganisms and cells)[6].
Opportunistic infection caused by Gram-positive cocci account for up to 90% of IE cases. Oral streptococci found in the mouth and gastrointestinal (GI) tract; Staphylococcus aureus and coagulase-negative staphylococci from the skin; and Enterococci (several species) spp. from the lower GI tract are commonly implicated[13–15]. Oral streptococcal cases of IE were previously the most common in the UK; however, nosocomial sources of infection with Staphylococcus aureus and Enterococci are now more frequently seen. This may be owing to increasing use of long-term intravenous lines, invasive procedures (e.g. hip replacement surgery), prosthetic devices (e.g. cardiac pacemaker, prosthetic valves) and immunosuppressive interventions (e.g. chemotherapy) resulting in greater numbers of staphylococcal and enterococcal bloodstream infections (a precursor of IE)[12]. Gram-negative bacilli such as the HACEK bacteria (Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae) account for 1–5% of IE cases; other Gram-negative pathogens — which cause a high proportion of all invasive infections — are rarely associated with IE. Fungal endocarditis, caused by yeast (Candida spp.) or by filamentous fungi (Aspergillus spp.), account for about 1% of IE cases, usually in post-surgical or immunocompromised patients in the presence of a prosthetic valve[8].
A small but possibly unrecognised proportion of patients will present with the clinical features of endocarditis with a negative blood culture[5]. This may be a consequence of antimicrobial administration prior to taking blood samples, the presence of fastidious microorganisms undetected by standard culturing techniques or non-infective endocarditis[5]. Serological testing can be carried out for Coxiella burnetii, Bartonella spp., Mycoplasma pneumonia, Brucella spp. and Legionella pneumophila, while a polymerase chain reaction test of the blood may identify Tropheryma whipplei, Bartonella spp. and some fungi[5,15]. Non-infective endocarditis aetiology may also be seen as a secondary complication of autoimmune or neoplastic conditions. Here, non-bacterial thrombotic endocarditis may develop, resulting in the formation of sterile vegetations[16].
Clinical features and diagnosis
The diagnosis of IE is inherently challenging owing to the highly variable and non-specific presentation for most patients. Presentation may be acute (less than one month), sub-acute (one to three months) or chronic (more than three months) depending on host and pathogen factors[5,13].
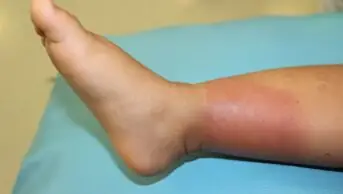
Fever with weight loss and reduced appetite are general symptoms present in up to 90% of IE patients. Heart murmurs — turbulent blood flow audible on stethoscope — are the most common cardiac manifestation, with new or existing murmurs heard in 85% of cases[13]. Disseminated infection, caused by embolic spread of fragmented vegetation from cardiac tissue, can result in severe extracardiac complications of IE, including cerebral, cutaneous or organ ischaemia. Cerebral abnormalities may be seen in up to 80% of patients on MRI imaging, but the majority are asymptomatic[2]. Cutaneous manifestations present as peripheral emboli, such as splinter haemorrhages and petechiae (i.e. pinpoint-sized spots of bleeding under the skin or mucous membranes) together with Roth spots, Osler nodes and Janeway lesions (see Figure)[5]. Splenomegaly (enlarged spleen) owing to direct septic emboli or seeding of the primary bacteraemia can be found in 35–60% of patients with new IE[2]. Unexplained heart failure and persistent bacterial infection without a clear source of infection may also alert to possible IE (see Table 1).
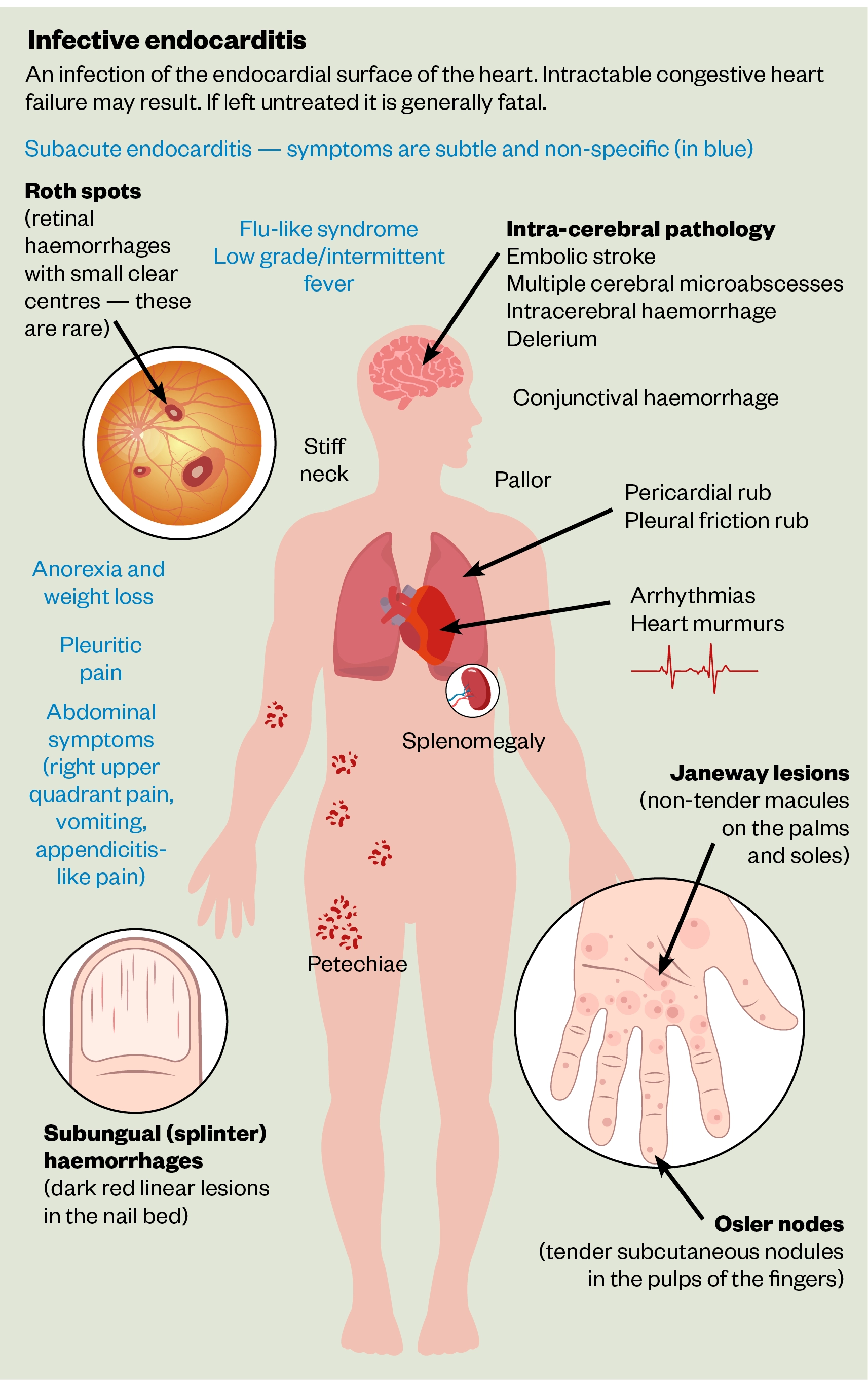
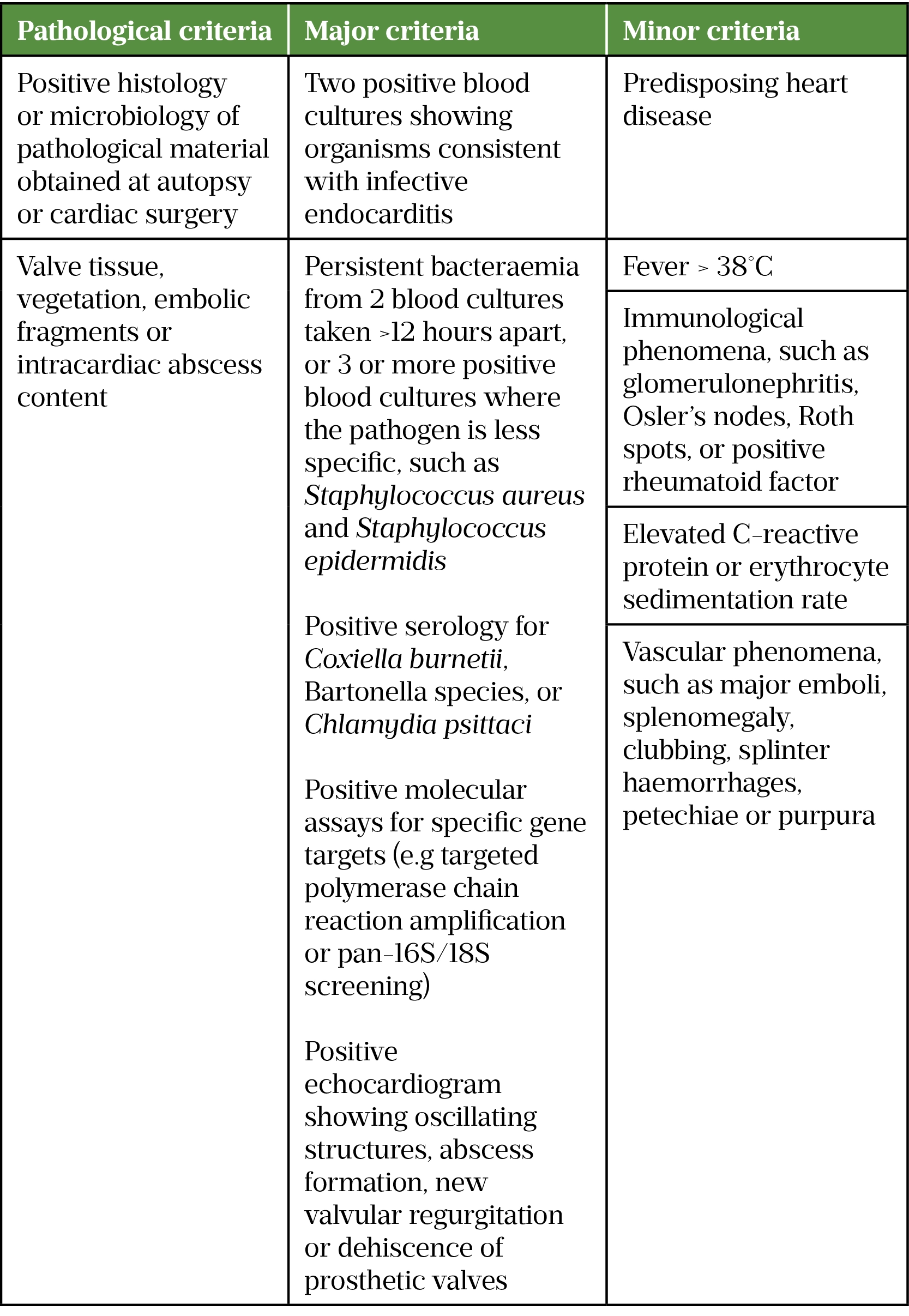
Diagnosis of IE relies on the modified Duke criteria, which combines clinical signs and symptoms, microbiological testing and cardiac imaging to predict infection. Definite IE is defined as meeting any pathological criteria, two major clinical criteria, one major criterion with three minor criteria, or five minor criteria. Possible IE is defined as meeting one major criterion with one minor criterion or three minor criteria (see Table 2)[17]. For confirmatory microbiological testing, three sets of blood cultures taken at 30-minute intervals prior to systemic antibacterial exposure are advised to improve the diagnostic yield from blood culture and positively identify the causative organism[6,12].
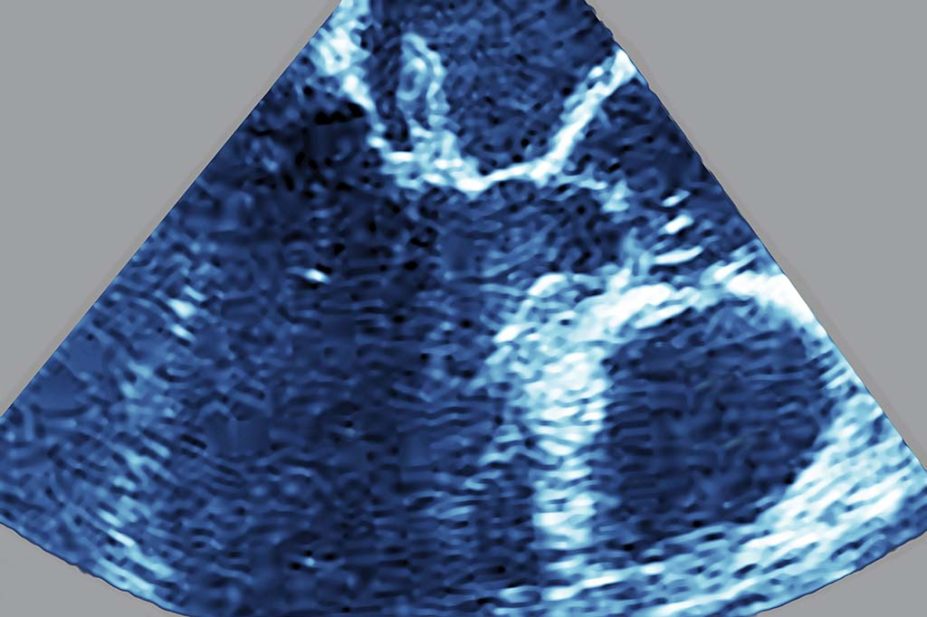
Echocardiography is the preferred imaging modality for patients with suspected IE. Its benefits include the identification of vegetation, assessing valvular function and predicting complications[12]. Transthoracic echocardiogram (TTE) is the initial technique of choice as it is safe, quick, and non-invasive. If a TTE is inconclusive, particularly in those with prosthesis, this necessitates the use of a transoesophageal echocardiogram (TOE). TOE has a higher degree of sensitivity when compared to a TTE but is more costly and invasive. Other diagnostic modalities can be used, including cardiac CT scan, positron emission tomography scanning and white blood cell scintigraphy, which are considered to reduce the rate of misdiagnosed IE in patients that fall into the “possible IE” category[5]. An echocardiogram should be considered in all patients with persistent bacteraemia where no source has been identified.
Management
A multidisciplinary approach to the management of patients with IE is advised, with input from cardiologists, cardiothoracic surgeons, infectious disease specialists and the pharmacy team. Patients with confirmed or highly probable IE should be initiated on empiric antibacterial therapy once blood cultures have been taken. In sub-acute presentation, it may be appropriate to delay initial therapy until microbiological confirmation of infection; however, early administration is necessary for acute presentations. Empirical antibacterial therapy should provide coverage against commonly expected pathogens — this should be tailored based on the presence of prosthetic valves and/or recent risk factors for infection, such as active intravenous drug use. The UK guidelines produced by the British Society for Antimicrobial Chemotherapy (BSAC) were last updated in 2004 and have been superseded for many acute UK trusts by the European Society of Cardiology (ESC) guidelines in 2015 (see Table 3)[5,18].
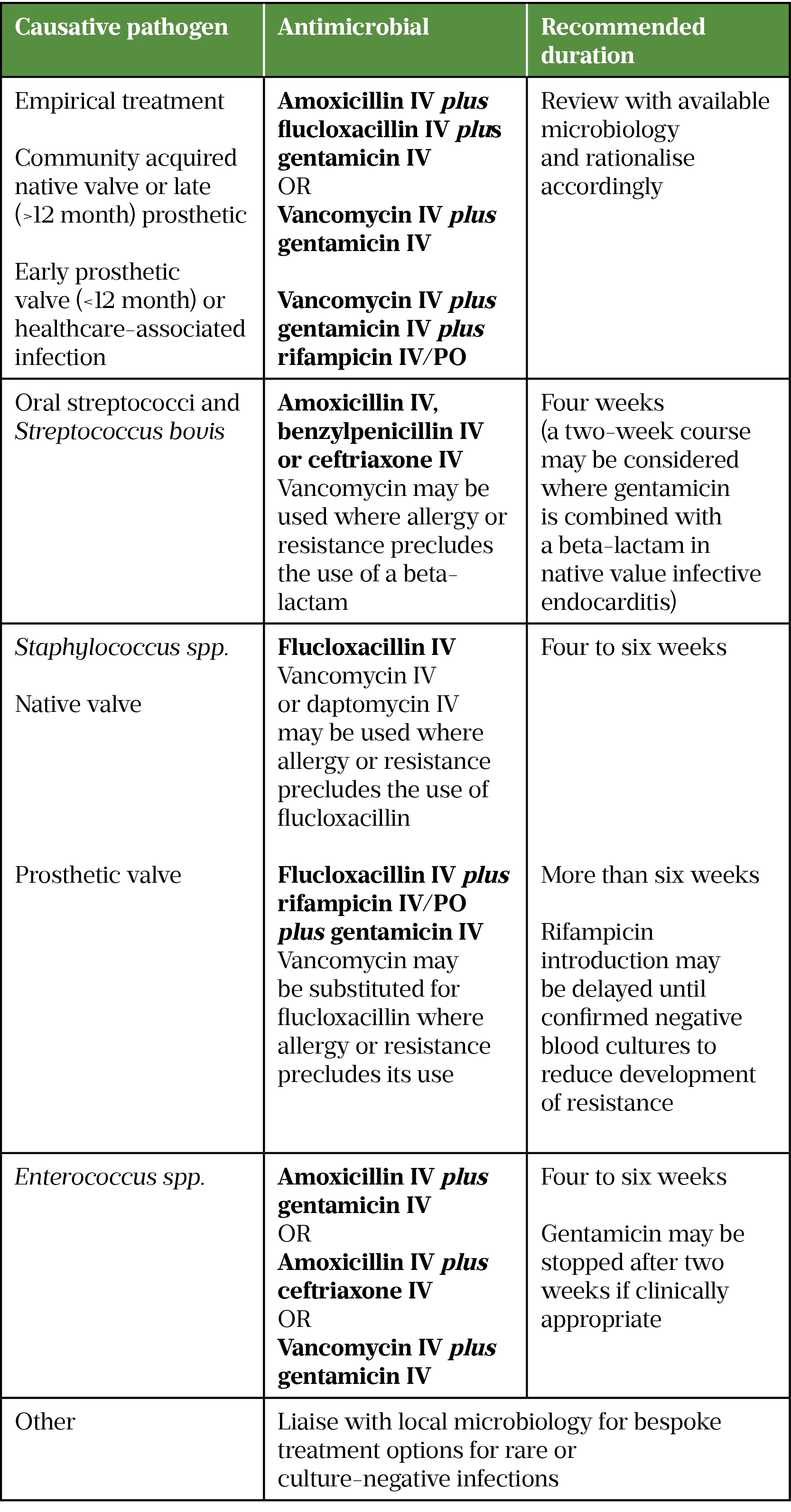
The updated ESC guidelines have resulted in a gradual change to the management of IE in the UK. There has been a move away from the use of synergistic gentamicin for many native valve infections; the risk of aminoglycoside toxicity (both nephro- and oto-toxicity) likely outweigh the perceived benefit[5]. The absolute risk of added toxicities is difficult to determine; patient risk factors (age, renal function, human leukocyte antigen (HLA) genetic profile) will increase these risks. There is still a role for gentamicin in empiric treatment (for the coverage of HACEK pathogens), as well treatment of enterococcal native valve IE and some of the prosthetic valve IE causative pathogens[5].
Small variations in dosing recommendations are seen between ESC and the legacy BSAC guidelines. Of interest, the ESC guidance recommends a once-daily aminoglycoside (gentamicin 3mg/kg) as an alternative to the multi-daily dosed option in BSAC guidelines (gentamicin 1mg/kg twice daily) for Gram-positive IE. High-dose beta-lactams are recommended when treating for intravascular-sourced infections, such as IE, and some suggested dosing by ESC guidelines may exceed the British National Formulary (BNF) recommendations (e.g. flucloxacillin IV 2g every 4 hours). Local trust policies should be consulted for further information. Dose adjustments for extremes of renal and hepatic dysfunction may be required in line with product licence or specialist renal dosing resources.
ESC recommends the use of ceftriaxone, as an alternative to gentamicin, combined with amoxicillin for the treatment of Enterococcal faecalis (E. faecalis) IE. While cephalosporins, such as ceftriaxone have no intrinsic activity against enterococci when used alone, combination use with amoxicillin allows saturation of penicillin-binding proteins with dual beta-lactams that potentiate the activity of the penicillin[19]. This provides an alternative to prolonged (two to six weeks) aminoglycoside therapy, where there is high level aminoglycoside resistance to E. faecalis or where aminoglycosides are not suitable (e.g. patients at risk of nephron- and/or oto-toxicity)[5].
Prolonged (two to six weeks) antibacterial therapy is advised for IE treatment (see Table 3). Initially, therapy is intravenously administered and the pharmacy team play an active role in the facilitation of continuous treatment. The use of out-patient parenteral antimicrobial therapy (OPAT) may assist continued intravenous therapy post-discharge. Intravenous antimicrobials treatment through once-daily administration or 24-hour infusions via elastomeric pumps can facilitate early discharge through the OPAT service. Alternatively, oral therapies may be utilised, where available, if the patient is clinically stable and has a viable oral treatment option. The recent randomised control trial Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis (POET) evaluated oral versus parenteral antimicrobial therapy, following ten-day minimum intravenous therapy in IE patients[20]. Clinical outcomes after six months demonstrated similar outcomes in patients treated with oral and parenteral therapy[20]. This has enabled the IE multidisciplinary teams to consider oral de-escalation in patients with stable haemodynamic features (e.g. afebrile, normotensive and no evidence of cardiac instability) and no surgical intervention required to facilitate early discharge. Ongoing patient monitoring can continue through weekly out-patient clinic assessments.
Surgical interventions to remove infected vegetations or repair cardiac structure may be indicated in IE with valvular dysfunction precipitating heart failure; in patients with persistent bacteraemia despite targeted antimicrobial therapy; and patients with risk of embolisation (mobile vegetation >10mm observed)[2,21].
Prevention
Antibacterials have been prescribed to prevent IE for many decades. National Institute for Health and Care Excellence (NICE) guidance no longer recommends the routine use of prophylaxis in patients undergoing dental, gastrointestinal, gento-urinary or respiratory procedures, owing to a lack of robust supporting evidence[22]. Some controversy remains following this change of practice and, while there are increasing numbers of microbiologically confirmed IE cases, these cannot be clearly linked with this intervention. Good oral hygiene is advised for at-risk patients including those with:
- Acquired valvular heart disease with stenosis or regurgitation;
- Hypertrophic cardiomyopathy;
- Previous IE;
- Structural congenital heart disease, including surgically corrected or palliated structural conditions, but excluding isolated atrial septal defect, fully repaired ventricular septal defect or fully repaired patent ductus arteriosus;
- Closure devices that are judged to be endothelialised and valve replacement[22].
Pharmacists should encourage patients, especially those in a high-risk category, to brush all tooth surfaces at least twice per day and have regular dental check-ups[23].
Best practice points for pharmacists
- Routine prophylaxis for IE is not recommended for patients undergoing interventional procedures as per NICE guidance;
- An echocardiogram should be carried out in patients with persistent staphylococcus/enterococcus bacteraemia;
- Pharmacists play a vital role in delivery of OPAT/intravenous and complex oral antimicrobial therapy;
- Doses of antimicrobials used for the treatment of IE vary from common indications and may exceed BNF dose recommendations.
- 1Mylonakis E, Calderwood SB. Infective Endocarditis in Adults. N Engl J Med. 2001;345:1318–30. doi:10.1056/nejmra010082
- 2Hoen B, Duval X. Infective Endocarditis. N Engl J Med. 2013;368:1425–33. doi:10.1056/nejmcp1206782
- 3Mostaghim AS, Lo HYA, Khardori N. A retrospective epidemiologic study to define risk factors, microbiology, and clinical outcomes of infective endocarditis in a large tertiary-care teaching hospital. SAGE Open Medicine. 2017;5:205031211774177. doi:10.1177/2050312117741772
- 4Habib G. Management of infective endocarditis. Heart. 2006;92:124–30. doi:10.1136/hrt.2005.063719
- 5Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J. 2015;36:3075–128. doi:10.1093/eurheartj/ehv319
- 6Cahill TJ, Prendergast BD. Infective endocarditis. The Lancet. 2016;387:882–93. doi:10.1016/s0140-6736(15)00067-7
- 7Njuguna B, Gardner A, Karwa R, et al. Infective Endocarditis in Low- and Middle-Income Countries. Cardiology Clinics. 2017;35:153–63. doi:10.1016/j.ccl.2016.08.011
- 8Pant S, Patel NJ, Deshmukh A, et al. Trends in Infective Endocarditis Incidence, Microbiology, and Valve Replacement in the United States From 2000 to 2011. Journal of the American College of Cardiology. 2015;65:2070–6. doi:10.1016/j.jacc.2015.03.518
- 9Hospital Episode Statistics. NHS Digital. 2022.https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed Jul 2022).
- 10Thornhill MH, Dayer MJ, Nicholl J, et al. An alarming rise in incidence of infective endocarditis in England since 2009: why? The Lancet. 2020;395:1325–7. doi:10.1016/s0140-6736(20)30530-4
- 11Kadri AN, Wilner B, Hernandez AV, et al. Geographic Trends, Patient Characteristics, and Outcomes of Infective Endocarditis Associated With Drug Abuse in the United States From 2002 to 2016. JAHA. 2019;8. doi:10.1161/jaha.119.012969
- 12Chen E, Smith B, Marschalk N, et al. Epidemiology and pathophysiology of infective endocarditis. In: Kilic A, ed. Infective Endocarditis. London: : Academic Press 2022. 1–23.
- 13Tornos P, Gonzalez-Alujas T, Thuny F, et al. Infective Endocarditis: The European Viewpoint. Current Problems in Cardiology. 2011;36:175–222. doi:10.1016/j.cpcardiol.2011.03.004
- 14Tleyjeh IM, Steckelberg JM. Changing epidemiology of infective endocarditis. Curr Infect Dis Rep. 2006;8:265–70. doi:10.1007/s11908-006-0070-0
- 15Vogkou CT, Vlachogiannis NI, Palaiodimos L, et al. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis. 2016;35:1227–45. doi:10.1007/s10096-016-2660-6
- 16Hurrell H, Roberts-Thomson R, Prendergast BD. Non-infective endocarditis. Heart. 2020;106:1023–9. doi:10.1136/heartjnl-2019-315204
- 17Li JS, Sexton DJ, Mick N, et al. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clinical Infectious Diseases. 2000;30:633–8. doi:10.1086/313753
- 18Gould FK, Denning DW, Elliott TSJ, et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. Journal of Antimicrobial Chemotherapy. 2011;67:269–89. doi:10.1093/jac/dkr450
- 19Mirna M, Topf A, Schmutzler L, et al. Time to abandon ampicillin plus gentamicin in favour of ampicillin plus ceftriaxone in Enterococcus faecalis infective endocarditis? A meta-analysis of comparative trials. Clin Res Cardiol. 2021. doi:10.1007/s00392-021-01971-3
- 20Iversen K, Ihlemann N, Gill SU, et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N Engl J Med. 2019;380:415–24. doi:10.1056/nejmoa1808312
- 21Prendergast BD, Tornos P. Surgery for Infective Endocarditis. Circulation. 2010;121:1141–52. doi:10.1161/circulationaha.108.773598
- 22Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. National Institute for Health and Excellence. 2016.https://www.nice.org.uk/guidance/cg64/chapter/update-information (accessed Jul 2022).
- 23Chapter 8: Oral hygiene. UK Government. 2021.https://www.gov.uk/government/publications/delivering-better-oral-health-an-evidence-based-toolkit-for-prevention/chapter-8-oral-hygiene (accessed Jul 2022).