Shutterstock.com
After reading this article, you should be able to:
- Understand the difference between MRSA colonisation and infection;
- Understand how pharmacists and pharmacy technicians can optimise the care of a patient with MRSA;
- Understand how antimicrobial stewardship and infection prevention and control measures can reduce the transmission and emergence of MRSA;
- Effectively counsel patients and carers about MRSA decolonisation regimens or antibiotics to support compliance.
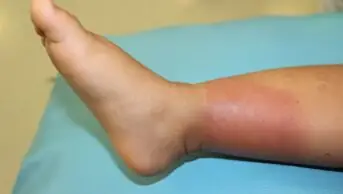
Staphylococcus aureus (S. aureus) is a common gram-positive bacterium that colonises human skin and mucous membranes[1]. It is usually a harmless member of the skin flora; however, infection can occur if there is a breach to the skin or mucosal barrier, followed by tissue invasion and damage[2]. While it frequently causes skin and soft tissue infections (accounting for up to 80% of pathogen-positive specimens), S. aureus can more rarely result in life-threatening infections, such as bacteraemia (i.e. blood stream infection), with population incidence estimates ranging from 10–30 per 100,000 person-years among high income countries[3–5].
Colonisation and infection are two different states, and it is important to differentiate between them. When bacteria exist within an environment such as the mouth, skin or airway without causing disease, the host is said to be colonised. If the pathogen invades the host’s tissues and multiplies, the host is infected with the pathogen[5].
Most strains of S. aureus are sensitive to methicillin, a penicillin antibiotic. Methicillin use was replaced with alternative penicillin antibiotics with better stability and side effect profiles around 1982; however, the terms methicillin-sensitive or methicillin-resistant continue to be used to describe the susceptibility of S. aureus to penicillin[6–8].
A 2024 report highlighted that there is an increase of 16.6% from April 2022 to March 2023 period in comparison to previous year. This coincides with an increase in hospital-onset cases was observed during the same year, with the rate increasing from 0.7 cases per 100,000 bed-days to 0.8[9]. Comparing January to March 2023 with the same period in 2019 (January to March 2019), which represents a more typical year before the COVID-19 pandemic, a 17.3% increase was seen in all reported counts of cases from 179 to 210 cases. These trends indicating the return of MRSA rates to pre-pandemic levels[10].
MRSA bacteria are easily transmitted in healthcare settings and infections have limited treatment options[11,12]. Prevention is therefore important to reduce the impact on patient outcomes and length of hospital stays, and healthcare professionals should understand the diagnosis, screening, and management of MRSA-colonised and MRSA-infected patients.
Pharmacists and pharmacy staff can reduce the spread of MRSA by encouraging prudent antimicrobial use, providing education on local MRSA policies and adherence to local infection prevention and control (IPC) guidance[13].
Epidemiology
In the 1990s, MRSA accounted for 34% of S. aureus bacteraemia isolates in UK microbiology laboratories[14]. In 1998, national guidelines for controlling MRSA were published, which covered screening, isolation and hand hygiene, bringing standard infection control policies into healthcare settings[11]. Despite these measures, by 2002, the UK had the second highest MRSA rate in Europe, with an estimated 44% of S. aureus isolates identified as MRSA[15]. Mandatory surveillance of MRSA was introduced in 2001 and was enhanced in 2007 to collect demographic, clinical and epidemiological information on each case[9]. Since then, the incidence of MRSA bacteraemia has reduced significantly (see Figure 1)[10].
In 2013, NHS England introduced a “zero tolerance” approach to MRSA bacteraemia[16]. This approach resulted in it being compulsory to report cases of MRSA bacteraemia to the UK Health Security Agency and conduct a root cause analysis (RCA) (also known as a post-infection review) for each case. The RCA panel consists of all organisations involved in the patient’s care pathway to jointly identify any lapses of care and areas for improvement, as well as agreeing on actions to prevent recurrence[17]. The main components examined in an RCA are contributing factors in screening for colonisation, devices used by patient, antimicrobial therapy, skin integrity, transmission, hand hygiene, environment, and organisational and governance issues[17].
From 2018, MRSA bacteraemia reporting has only been mandatory for trusts and clinical commissioning groups with the highest MRSA rates[16]. Pharmacists in primary and secondary care should support the preparation of RCAs by reviewing the appropriateness of antimicrobial prescribing (e.g. choice, dosage and duration) as well as providing information on MRSA decolonisation therapy, depending on patient eligibility. The panel will ensure that learning and good practice from reviews are shared with wider clinical teams. For example, if a contributing factor is inappropriate antimicrobial prescribing, the pharmacist on the panel should cascade this within the pharmacy department and clinical team who look after the patient, and stipulate any actions to prevent recurrence. The department will also be expected to undertake an audit on antimicrobial prescribing[17].
Infection, prevention and control measures
MRSA is now considered endemic in healthcare settings, especially in areas deemed high-risk (e.g. intensive care, vascular, burns and orthopaedic units). Patients with indwelling medical devices, trauma or surgical wounds are deemed at greater risk of colonisation or infection[18,19]. The most common route of MRSA transmission in healthcare settings is through healthcare workers, who acquire the organism from direct contact with patients who are either colonised or infected with MRSA[11]. Rarer routes of transmission include touching patient’s skin, or via inanimate objects or environmental surfaces (e.g. clothing, bedding, the area around a patient’s bed or a patient’s bathroom)[19]. Effective hand hygiene is the cornerstone of good IPC measures. Hand washing with soap and water, or use of alcohol sanitiser, and covering cuts or abrasions with a waterproof dressing are all advised. Pharmacists and their teams should be aware of the five moments of hand hygiene (see Figure 2), follow the IPC recommendations in their daily practice and challenge others who are not adhering to this guidance[20]. Other IPC strategies for managing patients colonised or infected with MRSA include barrier nursing the patient with single-use gloves and aprons, and isolating the patient in a side room (whenever possible)[19].
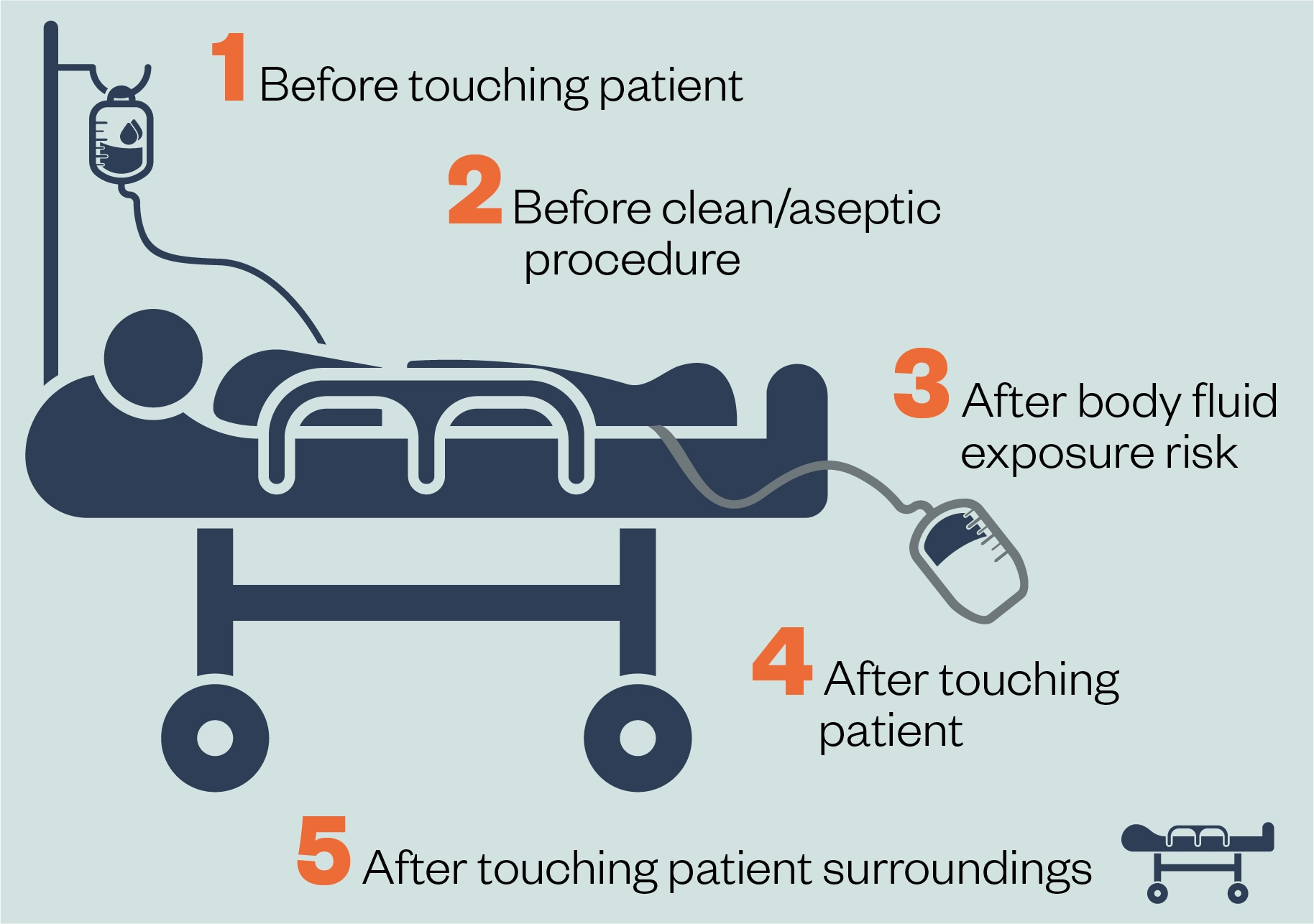
Reproduced from the World Health Organization’s guidelines on hand hygiene in healthcare[20]
Detection of patients colonised or infected with MRSA
Detection of colonised patients
MRSA colonisation is detected through microbiological screening. Details of patients recommended for screening can be seen in Box 1[18,21]. Some trusts may have additional patient groups who require screening. Local risk assessments should be used to define other potential high MRSA-risk units/specialties (e.g. transplant, neonatal) and units with a history of high endemicity of MRSA; therefore, local policy should be consulted[21].
Box 1: Department of Health and Social Care and National Institute for Health and Care Excellence recommendations for MRSA screening
Secondary care:
- All patients admitted to high-risk specialties*;
- All patients previously identified as colonised with or infected by MRSA.
Primary care:
- Patients presenting with suspected MRSA infection[18,21].
* Vascular, renal/dialysis, neurosurgery, cardiothoracic surgery, haematology/oncology/bone marrow transplant, orthopaedics/trauma, and all intensive care units, high dependency units and coronary care units.
Microbiological screening often involves nasal screening because the anterior nares are the most frequently colonised site. However, areas of damaged skin should also be screened. Recommendations for further screening sites (e.g. groin, axilla, and perineum) vary between national and local guidelines; therefore, local policy should be consulted[22,23].
Various microbiological methods may be used to detect MRSA in screening specimens. The first laboratory stage involves selectively culturing methicillin-resistant strains (via direct or enrichment culture, most frequently with a chromogenic selective MRSA medium, or, alternatively, molecular methods). This is followed by the ‘identification’ stage, which must, as a minimum, identify a S.aureus species and demonstrate resistance to the antibiotic cefoxitin prior to reporting the result[24].
Often, there is an alert system within the organisation that allows healthcare professionals to identify patients with a history of MRSA colonisation or infection; it is important to be familiar with the local alert system when reviewing patients’ antimicrobial therapy to ensure that MRSA decolonisation is prescribed when indicated.
Detection of MRSA-infected patients
To detect MRSA, a clinical sample must be taken from the patient and sent to the laboratory. The sample type taken may correlate specifically with the suspected clinical infection syndrome or it may have been taken as part of a ‘septic screen’. For example, blood cultures must be taken when identifying suspected sepsis, additional information on which can be found here[25].
S. aureus can be cultured from a variety of samples, including blood, pus, sputum, urine and other specimens, such as joint fluid aspirates, depending on the suspected source of infection[26]. Organisms that have grown and been identified as S. aureus will undergo antimicrobial susceptibility testing, which may then identify the organism as MRSA[27,28].
MRSA infection is clinically indistinguishable from MSSA (methicillin-susceptible S. aureus) infection, however, it has a stronger association with hospital-acquired infections than MSSA and case fatality ratios tend to be higher for MRSA bacteraemia compared with MSSA bacteraemia[29,30]. MRSA infection is more likely in patients with:
- Recent hospitalisation or invasive devices, such as urinary catheters;
- Previous (or current) MRSA infection or colonisation;
- Residence in long-term care facilities;
- Previous antibiotic use;
- Intravenous drug use;
- No response to first-line antibiotics;
- Recurrent skin or soft tissue infections, or a chronic non-healing ulcer, especially if purulent[18].
What action should be taken when infection is suspected?
Depending on the clinical infection syndrome suspected and the severity of clinical illness, empirical antibiotics may be started based on local microbiological guidelines for the suspected syndrome. Ideally, clinical sampling should be performed before starting antibiotics to ensure better microbiological culture yield. In patients known to be colonised with MRSA or thought to be at high risk of MRSA infection, clinicians may opt for antibiotics that cover MRSA prior to receiving any culture/sensitivity results. Where laboratory confirmation has occurred and MRSA is believed to be the most likely culprit pathogen, effective treatment regimens should cover MRSA.
Nasal and skin decolonisation
MRSA decolonisation is not routinely required for asymptomatic carriers of MRSA in the community. However, if a patient colonised with MRSA is undergoing a high-risk surgery (see Box 1) they should receive skin and nasal decolonisation to reduce the MRSA burden. This can minimise the risk of surgical site infection and reduce transmission to other patients[18,31].
The successful eradication of MRSA is not possible every time; however, any reduction in bacterial load will reduce the risk of endogenous infection or transmission to other patients[32]. Local policy should be observed for re-screening post-decolonisation and for repeated decolonisation.
In clinical practice, the choice and duration of skin and nasal products will vary with local guidance and should consider:
- The type of procedure;
- Individual patient risk factors, including allergy;
- Patients with carers or live-in relatives who have a severe allergy to any of the product ingredients (specifically arachis oil), as they should not be given the respective products;
- The risk of severe chemical injuries with the use of chlorhexidine (both alcohol-based and aqueous solutions) in preterm babies (an alternative is an alcohol-based solution of povidone-iodine);
- The potential impact of infection on the individual patient, including the cost of managing the infection;
- Local formulary and resistance pattern[33].
Nasal decolonisation
The National Institute for Health and Care Excellence (NICE) recommends nasal mupirocin ointment in combination with a chlorhexidine body wash before procedures in which S. aureus can potentially cause surgical site infection[33]. Neomycin/chlorhexidine nasal cream can also be used for patients with mupirocin resistant MRSA or allergy to an ingredient in mupirocin ointment (see Box 2 for dosing information)[34]. Counselling points for the administration of nasal ointments and creams can be found here.
Box 2: Dosing information for nasal decolonisation products
Mupirocin nasal ointment:
- Apply to both nostrils three times per day for five days[34,35].
Neomycin/chlorhexidine nasal cream:
- Apply to both nostrils four times per day for ten days;
- Contains arachis oil (peanut oil) and should not be applied by patients or carers with known allergy to peanut. Patients or carers with soya allergy should also avoid this product, owing to a possible relationship between peanut allergy and soya allergy. The manufacturer is in the process of removing of arachis oil, but some will remain in circulation until 2025[34,36,37].
These are typical instructions for these nasal products but there is variation in local policy.
Skin decolonisation
Skin cleansers are used in combination with nasal ointment for MRSA skin decolonisation; chlorhexidine 4% skin cleanser is the most common[38]. Individual product information should be consulted for advice on how to use the products, including contact time.
Cleansers should not be used on cuts or areas of broken skin. It is important to consider the side effects of the cleanser; for example, chlorhexidine 4% may cause irritation or chemical burns in patients with existing skin conditions and in preterm babies[33,39].
The fragrance in one of the chlorhexidine 4% cleanser formulations has recently changed, and the product now contains soya oil. The manufacturer now advises against using this product for patients with peanut and soya allergies[38].
The most common side effect of nasal and skin products are skin reactions. Patients should stop using the product and inform relevant healthcare professionals if this happens[34].
As part of decolonisation counselling, patients should be advised:
- How to use the products;
- The importance of using clean wash cloths and clean dry towels;
- To change into clean clothing after the wash;
- To change bedding daily while using the decolonisation products;
- What to do if allergy, adverse reaction or side effects occur[34].
Surgical prophylaxis for MRSA-colonised patients
Antibiotic prophylaxis should be given to patients undergoing any surgery that is not defined as ‘clean non-prosthetic uncomplicated surgery’, according to NICE guidelines[33]. For patients who are MRSA-colonised, the prophylactic antibiotic(s) chosen must cover MRSA in addition to the standard spectrum of cover, which usually includes both gram-positive and gram-negative cover but excludes MRSA.
Local antibiotic guidelines for surgical prophylaxis should therefore always provide an alternative (to the standard) regimen used for MRSA-colonised patients. Most commonly, intravenous teicoplanin is used.
It is important to ensure that the indication is clearly stated when prescribed, as surgical prophylaxis (including MRSA cover for a colonised patient) may require a single dose, avoiding the incorrect assumption that a treatment course may be required. This is an important opportunity to prevent possible overuse of antibiotics.
Treatment of MRSA infection
Vancomycin and teicoplanin (both given parenterally) remain the antibiotics of choice for treatment of MRSA infection[12]. They are glycopeptide antibiotics that are active against most gram-positive microorganisms. Vancomycin has a narrow therapeutic range (trough level of 10–20mg/L). For deep-seated infection, such as meningitis and bone and joint infection, a trough level of 15–20mg/L is recommended to achieve clinical effectiveness[12,40]. Levels above this range can cause toxicity — including nephrotoxicity and ototoxicity — while sub-therapeutic levels can lead to treatment failure and contribute to antimicrobial resistance (AMR)[40,41]. Consequently, therapeutic drug monitoring (TDM) of vancomycin and appropriate dose adjustments are paramount, especially for patients with a life-threatening MRSA infection. Teicoplanin has a wider therapeutic range (trough levels vary based on indication, see guidelines for more information) and the use of TDM for this drug varies between trusts[41].
Pharmacists should be able to undertake TDM to optimise a patient’s therapy to minimise the risk of adverse reactions[12,13]. Local prescribing guidelines should be used for dosing guidance and target therapeutic levels for vancomycin and teicoplanin.
Other antibiotics used for the management of MRSA are selected based on the organism’s sensitivity pattern, but may include linezolid, co-trimoxazole, daptomycin, clindamycin and rifampicin, ciprofloxacin and delafloxacin[12,13]. Please note, the MHRA has released several drug safety updates for fluroquinolone antibiotics, so they must now only be prescribed when other commonly recommended antibiotics are inappropriate[42]. For each of these antibiotics, the cautions, contraindications, interactions and monitoring (where appropriate) should be considered. The updated 2021 BSAC guidelines recommend treatment regimens for various types of MRSA infection[12].
Adherence is vital and patients should be involved in the decision-making process. Information relating to adherence advice can be found here[43].
Antimicrobial stewardship and MRSA
Globally, around 700,000 deaths each year are associated with AMR and, without intervention, mortality is estimated to reach 10 million by 2050[44]. A multidisciplinary approach is necessary to preserve the effectiveness of antimicrobials and reduce the transmission of infection[13,45,46]. Antimicrobial stewardship involves selecting an appropriate antimicrobial, and optimising its dose and duration while minimising toxicity is crucial. Pharmacists and pharmacy technicians in all sectors should be able to confidently review the appropriateness of antimicrobial prescriptions to prevent AMR. More information on how this can be done in practice can be found in ‘How to evaluate the clinical appropriateness of an antimicrobial’. Box 3 explores this role and outlines best practice in the context of MRSA.
Box 3: The role of pharmacists and pharmacy technicians in antimicrobial stewardship in the context of MRSA
- Follow IPC advice when reviewing MRSA patients to reduce transmission of the organism;
- Ensure that asymptomatic patients with MRSA colonisation do not receive unnecessary decolonisation or treatment for MRSA infection;
- Support treatment selection — for example, through:
- The choice of MRSA decolonisation therapy for an MRSA-colonised patient;
- The choice of surgical prophylaxis antibiotics for an MRSA-colonised patient;
- The choice of antibiotic for an MRSA-infected patient.
- Ensure the appropriate use of MRSA decolonisation products for the appropriate duration;
- For antibiotics prescribed in MRSA bacteraemia, ensure that the continuing need for antibiotics is reviewed regularly with documented actions to stop, continue, or change antimicrobial treatment;
- Support dose optimisation by accounting for patient factors (e.g. renal function) and considering therapeutic drug monitoring where appropriate;
- Counsel patients and carers about the MRSA decolonisation regimen or antibiotics to support compliance with regimens. This should include advice about finishing the course and the disposal of unused medication[45–47].
Summary
MRSA bacteria are easily transmitted in healthcare settings and have significant impact on patient outcomes, length of hospital stay and healthcare cost. It is therefore important for healthcare professionals to understand the diagnosis, screening and management of MRSA-colonised and MRSA-infected patients, in combination with IPC guidance, and apply these to day-to-day practice. Pharmacy professionals should familiarise themselves with their local MRSA policy and alert system so that they can identify patients that require prompt decolonisation or review of their antibiotic treatment.
This article has been reviewed and updated by co-author Bee Yean Ng to ensure it remains relevant, following its original publication in April 2022.
- 1Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. The Lancet Infectious Diseases. 2005;5:751–62.
- 2Moffarah AS, Al Mohajer M, Hurwitz BL, et al. Skin and Soft Tissue Infections. Microbiol Spectr. 2016;4.
- 3Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis. 2013;13.
- 4Laupland KB, Lyytikäinen O, et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clinical Microbiology and Infection. 2013;19:465–71.
- 5Colonization and infection. CEJU. 2014;67.
- 6Approved Drug Products with Therapeutic Equivalence Evaluations | Orange Book. US Food and Drug Administration. 2022. https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-therapeutic-equivalence-evaluations-orange-book (accessed April 2022)
- 7Newsom SWB. MRSA–past, present, future. JRSM. 2004;97:509–10.
- 8Castle SS. Methicillin. xPharm: The Comprehensive Pharmacology Reference. 2007;1–4.
- 9Annual epidemiological commentary: Gram-negative bacteraemia, MRSA bacteraemia, MSSA bacteraemia and C. difficile infections, up to and including financial year April 2022 to March 2023. UK Health Security Agency. 2024. https://www.gov.uk/government/statistics/mrsa-mssa-and-e-coli-bacteraemia-and-c-difficile-infection-annual-epidemiological-commentary/annual-epidemiological-commentary-gram-negative-mrsa-mssa-bacteraemia-and-c-difficile-infections-up-to-and-including-financial-year-2022-to-2023#epidemiological-analysis-of-staphylococcus-aureus-bacteraemia (accessed June 2024)
- 10Mandatory MRSA, MSSA, Gram-negative bacteraemia and C. difficile infections data (up to January to March 2023). UK Health Security Agency. 2024. https://www.gov.uk/government/statistics/mrsa-mssa-gram-negative-bacteraemia-and-cdi-quarterly-report/quarterly-epidemiological-commentary-mandatory-gram-negative-bacteraemia-mrsa-mssa-and-c-difficile-infections-data-up-to-january-to-march-2023 (accessed June 2024)
- 11Wilson J. Infection Control in Clinical Practice. 3rd ed. Paris: Bailliere Tindall 2006.
- 12Brown NM, Goodman AL, Horner C, et al. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): updated guidelines from the UK. JAC-Antimicrobial Resistance. 2021;3.
- 13Antimicrobial stewardship: From principles to practice. British Society for Antimicrobial Chemotherapy. 2018. https://www.bsac.org.uk/antimicrobialstewardshipebook/BSAC-AntimicrobialStewardship-FromPrinciplestoPractice-eBook.pdf (accessed June 2024)
- 14Reacher MH. Bacteraemia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: trend analysis. BMJ. 2000;320:213–6.
- 15Tiemersma EW, Bronzwaer SLAM, Lyytikäinen O, et al. Methicillin-resistantStaphylococcus aureusin Europe, 1999–2002. Emerg. Infect. Dis. 2004;10:1627–34.
- 16Mandatory Healthcare Associated Infection Surveillance: data quality statement. UK Health Security Agency. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/949223/Mandatory_healthcare_associated_infection_surveillance_data_quality_statement_2020.pdf (accessed April 2022)
- 17Guidance on the reporting and monitoring arrangements and post infection review process for MRSA bloodstream infections from April 2014 (version 2). Patient Safety Domain, NHS England. 2014. https://www.england.nhs.uk/patientsafety/wp-content/uploads/sites/32/2014/02/post-inf-guidance2.pdf (accessed June 2024)
- 18MRSA in primary care. National Institute for Health and Care Excellence. 2024. https://cks.nice.org.uk/topics/mrsa-in-primary-care/ (accessed June 2024)
- 19Collins F, Hampton S. Hand-washing and methicillin-resistant Staphylococcus aureus. British Journal of Nursing. 2005;14:703–7.
- 20SAVE LIVES — Clean Your Hands annual global campaign. World Health Organization. 2021. https://www.who.int/campaigns/world-hand-hygiene-day (accessed June 2024)
- 21Implementation of modified admission MRSA screening guidance for NHS (2014). Department of Health. 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/345144/Implementation_of_modified_admission_MRSA_screening_guidance_for_NHS.pdf (accessed June 2024)
- 22Warnke P, Frickmann H, Ottl P, et al. Nasal Screening for MRSA: Different Swabs – Different Results! PLoS ONE. 2014;9:e111627.
- 23Senn L, Basset P, Nahimana I, et al. Which anatomical sites should be sampled for screening of methicillin-resistant Staphylococcus aureus carriage by culture or by rapid PCR test? Clinical Microbiology and Infection. 2012;18:E31–3.
- 24UK Standards for Microbiology Investigations Identificationof Staphylococcus species, Micrococcus species and Rothia species. Public Health England and NHS. 2020. https://www.rcpath.org/profession/publications/standards-for-microbiology-investigations/identification.html?_gl=1*1k2rulk*_up*MQ..*_ga*MjEyMTY0Njc0OS4xNzE2NTYyNzA4*_ga_HT8YPHYFXZ*MTcxNjU2MjcwNy4xLjAuMTcxNjU2MjcwNy4wLjAuMA.. (accessed June 2024)
- 25Sepsis: recognition, diagnosis and early management. National Institute for Health and Care Excellence. 2024. https://www.nice.org.uk/guidance/ng51/resources/suspected-sepsis-recognition-diagnosis-and-early-management-pdf-1837508256709 (accessed June 2024)
- 26Tong SYC, Davis JS, Eichenberger E, et al. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin Microbiol Rev. 2015;28:603–61.
- 27Idelevich EA, Becker K. How to accelerate antimicrobial susceptibility testing. Clinical Microbiology and Infection. 2019;25:1347–55.
- 28UK Standards for Microbiology Investigations Investigation of blood cultures (for organisms other than Mycobacterium species). Public Health England and NHS. 2020. https://www.rcpath.org/profession/publications/standards-for-microbiology-investigations/bacteriology.html?_gl=1*1y3oeus*_up*MQ..*_ga*MjEyMTY0Njc0OS4xNzE2NTYyNzA4*_ga_HT8YPHYFXZ*MTcxNjU2MjcwNy4xLjAuMTcxNjU2MjcwNy4wLjAuMA (accessed June 2024)
- 29Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of Mortality Associated with Methicillin‐Resistant and Methicillin‐SusceptibleStaphylococcus aureusBacteremia: A Meta‐analysis. CLIN INFECT DIS. 2003;36:53–9.
- 30Blot SI, Vandewoude KH, Hoste EA, et al. Outcome and Attributable Mortality in Critically Ill Patients With Bacteremia Involving Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus. Arch Intern Med. 2002;162:2229.
- 31Surgical Skin Preparation Quality Improvement Resource. One Together UK. 2019. https://www.onetogether.org.uk/downloads/OneTogether%20Skin%20Prep%20QIR_2019.pdf (accessed June 2024)
- 32Sai N, Laurent C, Strale H, et al. Efficacy of the decolonization of methicillin-resistant Staphylococcus aureus carriers in clinical practice. Antimicrob Resist Infect Control. 2015;4.
- 33Surgical site infections: prevention and treatment. National Institute for Health and Care Excellence. 2020. https://www.nice.org.uk/guidance/NG125 (accessed June 2024)
- 34MRSA decolonisation patient information leaflet. Oxford University Hospitals NHS Trust 2021.
- 35Bactroban Nasal Ointment 2%. Electronic medicines compendium. 2019. https://www.medicines.org.uk/emc/product/1155/smpc (accessed June 2024)
- 36Naseptin Nasal Cream. Electronic medicines compendium. 2024. https://www.medicines.org.uk/emc/product/5524 (accessed June 2024)
- 37Naseptin Nasal Cream safety alert. Electronic Medicines Compendium. 2024. https://www.medicines.org.uk/emc/product/5524/safetyalert#about-medicine (accessed June 2024)
- 38Hibiscrub. Mölnlycke Health Care. 2024. https://www.molnlycke.co.uk/products-solutions/hibiscrub/ (accessed June 2024)
- 39Chlorhexidine solutions: reminder of the risk of chemical burns in premature infants. Medicines and Healthcare products Regulatory Agency. 2014. https://www.gov.uk/drug-safety-update/chlorhexidine-solutions-reminder-of-the-risk-of-chemical-burns-in-premature-infants (accessed June 2024)
- 40Vancomycin 1g Powder for Solution for Infusion. Electronic medicines compendium. 2022. https://www.medicines.org.uk/emc/product/8760 (accessed June 2024)
- 41Antimicrobial Reference Laboratory. NHS Severn Pathology North Bristol NHS Trust. 2023. https://www.nbt.nhs.uk/severn-pathology/pathology-services/antimicrobial-reference-laboratory/antimicrobial-reference-laboratory-resources (accessed June 2024)
- 42Fluoroquinolone antibiotics: must now only be prescribed when other commonly recommended antibiotics are inappropriate. Medicines and Healthcare products Regulatory Agency. 2024. https://www.gov.uk/drug-safety-update/fluoroquinolone-antibiotics-must-now-only-be-prescribed-when-other-commonly-recommended-antibiotics-are-inappropriate (accessed June 2024)
- 43Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. National Institute for Health and Care Excellence. 2009. https://www.nice.org.uk/guidance/cg76 (accessed April 2022)
- 44No time to wait: Securing the future from drug-resistant infections . World Health Organization. 2019. https://www.who.int/publications/i/item/no-time-to-wait-securing-the-future-from-drug-resistant-infections (accessed June 2024)
- 45Ratnaraja NVDV, Davies AP, Atkins BL, et al. Best practice standards for the delivery of NHS infection services in the United Kingdom. Clinical Infection in Practice. 2021;12:100095.
- 46Confronting antimicrobial resistance 2024–2029: The UK’s five-year national action plan. HM Government. 2024. https://www.gov.uk/government/publications/uk-5-year-action-plan-for-antimicrobial-resistance-2024-to-2029 (accessed June 2024)
- 47Antimicrobial stewardship: Start smart — then focus. UK Health Security Agency. 2023. https://www.gov.uk/government/publications/antimicrobial-stewardship-start-smart-then-focus (accessed June 2024)