Shutterstock.com
After reading this article, you should be able to:
- Recognise the importance of managing postoperative pain effectively;
- Recognise the multimodal approach to managing postoperative pain;
- Recognise the role pharmacy teams can play in ensuring optimal peri-operative pain management.
Acute pain is a multi-dimensional experience influenced by several factors, including a person’s psychological state[1–3]. Postoperative pain or post-surgical pain (PSP) is sub-divided into ‘acute’ and ‘chronic’ pain, with acute pain experienced in the first seven days postoperatively[4]. Acute injury caused by surgery triggers sensitisation of peripheral pathways, as well as emotional responses of anxiety and fear[1].
The prevention and management of PSP is an important responsibility for healthcare professionals and an established part of peri-operative care[1,2]. There is evidence that PSP is often poorly managed, with up to 40% of patients reporting severe pain that negatively impacts their recovery, such as the prevention of early mobilisation[2]. Poorly controlled PSP is associated with a prolonged duration of opioid use and higher cost burden to health services[5]. Opioids can play an important role in the management of moderate and severe acute pain; however, persistent postoperative opioid use (PPOU) is a concern, as it increases the likelihood of misuse and harm[6,7].
Although limited UK-based data is available, there is a pattern of increasing deaths involving opioids[8](see Figure 1). Worldwide trends on deaths attributable to opioids have also increased considerably, in particular in the United States, with one study demonstrating a 292% increase between 2001 and 2016[9].
Poorly controlled postoperative pain is a risk factor for persistent or chronic post-surgical pain (PPSP)[2]. PPSP, which is defined as occurring for more than three months, is estimated to affect 5–60% of patients after all types of surgery and is heavily influenced by psychosocial (e.g. anxiety and depression) and genetic factors (e.g. single nucleotide polymorphisms)[2,10].
This article will outline general principles for the management of postoperative pain, with specific focus on the role of pharmacy teams, both peri-operatively and during follow-up in a primary care setting.
Pre-operative discussions
Surgical pharmacists form a crucial part of a broader multidisciplinary team (MDT) at preoperative assessment — which also include anaesthetists, nurses and orthogeriatricians — and provide evidence-based preoperative management to ensure optimal recovery from surgery[11].
Preoperative assessment provides an ideal opportunity to identify patients who may present with complex postoperative analgesic requirements and may be at risk of PPSP and PPOU, such as those with pre-existing chronic pain that is poorly managed and high opioid use[12,13]. Chronic opioid use may also result in opioid induced hyperalgesia, an increased response to painful stimuli[13–15]. Discussing patients’ expectations and psychologically preparing them for surgery will allow for realistic goal setting[16]. Psychological preparation can include, but is not limited to, providing postoperative instructions for the patient at preoperative assessment[17]. Potential discussion points for postoperative pain management are listed in Box 1[16,18–20].
Box 1: Points that should be covered when discussing postoperative pain management
- Determination of the patient’s medication history, allergies and intolerances. This will identify the current analgesic regimen and allow for the identification of any potential interactions (e.g. increased risk of serotonin syndrome);
- When chronic pain is being treated, ensure that this is recognised and managed appropriately. If pre-existing chronic pain is overlooked, patients are at risk of withdrawal if opioid analgesics, in particular, are unintentionally omitted from the management plan. Where possible, refer patients to a pain specialist for complex pain cases or, if necessary, services that can manage deprescribing (e.g. Perioperative medicine for older patients undergoing surgery [POPS] clinics);
- The clinician should work alongside other healthcare professionals to identify any risk factors for opioid misuse (e.g. younger age and use of opioids prior to surgery). This may influence the choice of postoperative analgesia;
- Manage expectations of pain and what surgery will achieve;
- Ensure patients have supplies of over-the-counter (OTC) analgesia available, in preparation for discharge;
- Counsel patients on the side effects of opioids, including sedation and constipation. Patients can also be advised on available OTC laxatives.
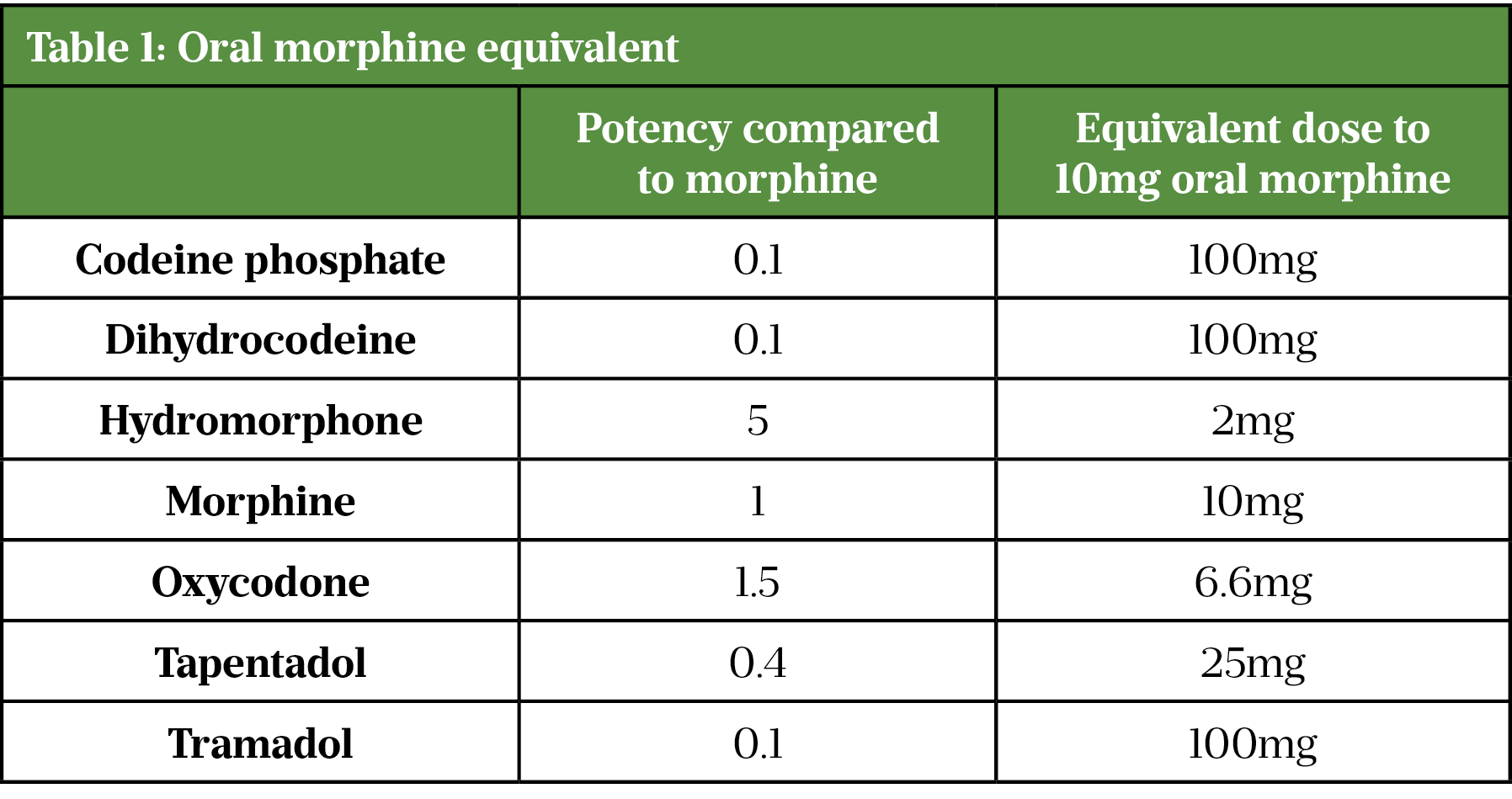
Patients with chronic pain who are managed on opioids should be optimised prior to surgery, especially in cases where the chronic pain will not improve regardless of the surgical procedure, and this may include weaning of opioids and the addition of opioid-sparing agents[6,16]. Opioid tolerance is more likely to occur in patients managed on opioids at doses of 60mg oral morphine equivalent (OME) and higher[13–15]. OME is based on the concept of doses of different opioids, with varying potency, producing similar analgesic effects[14].
Table 1 shows specific OMEs for different opioids. The table was initially designed for use in palliative care and should only be used as a screening tool in the context of peri-operative management, rather than to support opioid switching[16].
For those patients with complex pain needs (for example, managed on buprenorphine preoperatively) and other long-term opioid use prior to surgery, dedicated, specialist pain management is recommended[6,21]. Pharmacy teams can help optimise the PSP management plan by identifying patients managed on >60mg OME/day and other patients with complex needs (e.g. pregnancy), and following up with the proactive creation of a personalised acute pain management plan[6,22].
Assessment of pain
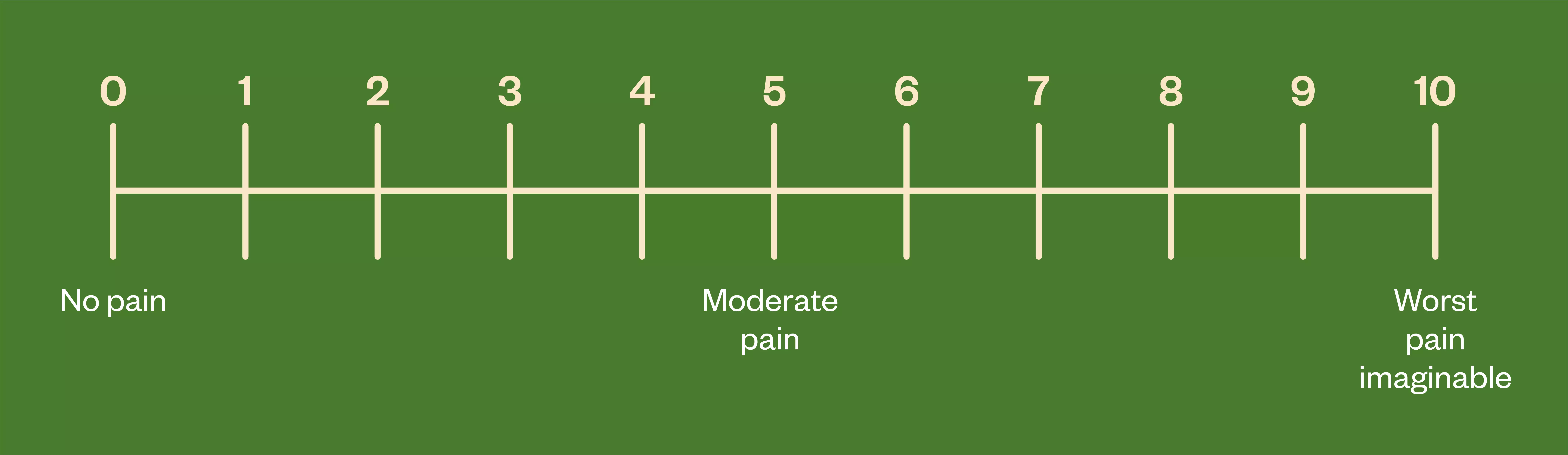
The visual analogue scale (VAS) is a commonly used tool to assess pain intensity in the immediate postoperative period[23]. VAS uses a scale from 0 (no pain) to 10 (worst pain imaginable) and can be used to assess pain over the previous 24 hours or over a longer time-period[23] (see Figure 2[24]).
Simple tools such as the VAS are frequently inadequate at describing the patient pain experience fully and each individual should be evaluated holistically, including surrogate assessment of pain management such as opioid consumption[1]. Numerical tools may be inappropriate at evaluating pain in certain patients, such as those with severe dementia, who are unable to self-report[1].
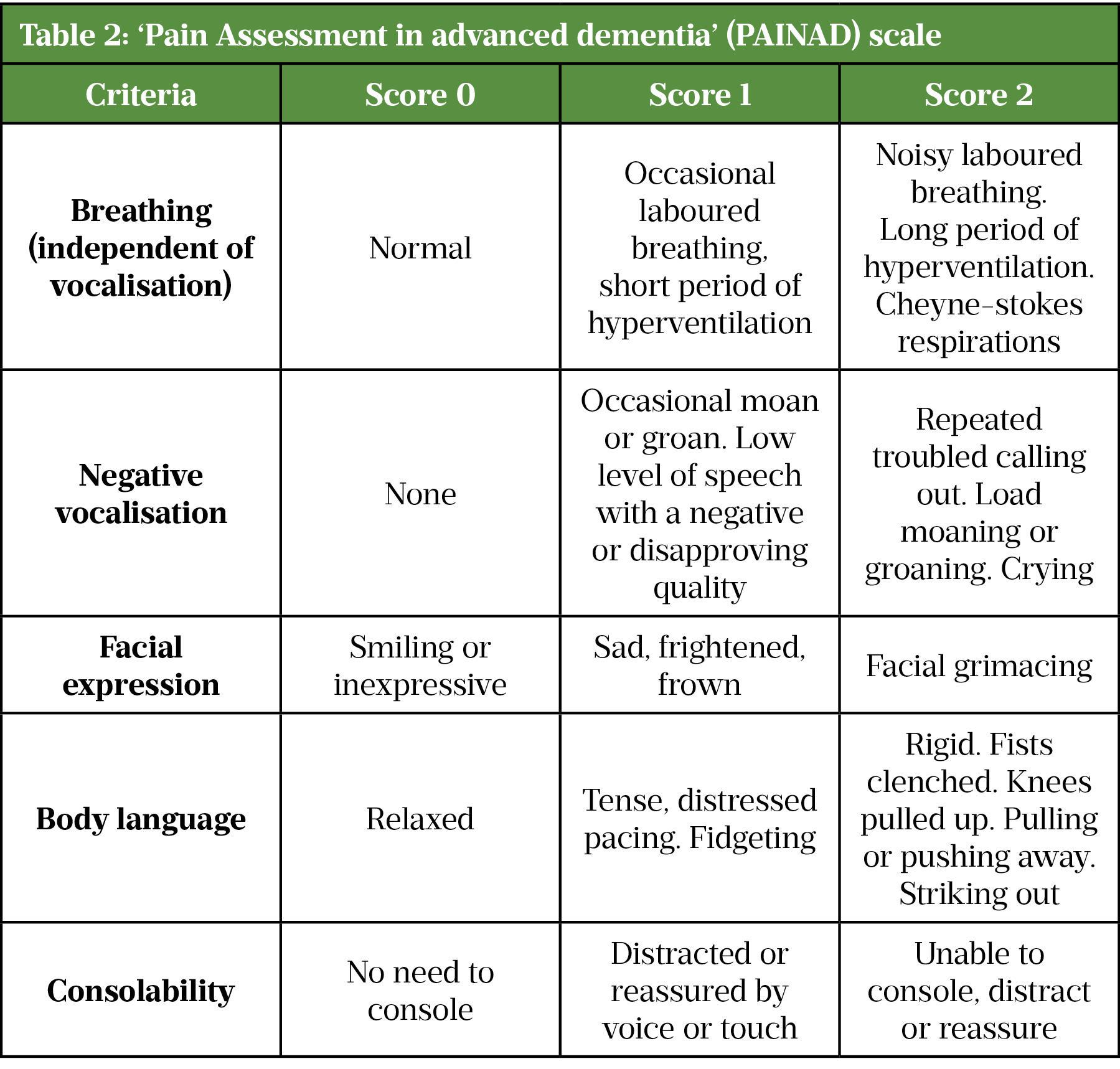
Other more specialised assessment tools have been developed in response. The Faculty of Pain Medicine Core Standards for Pain Management emphasise that appropriate assessment tools should be available for adults, children and those with dementia and learning difficulties[25]. Tools such as the Critical Care Pain Observation Tool (CPOT) and the Pain in Advanced Dementia Scale (PAINAD) have been developed and have demonstrated positive results[1,26]. Specific assessment tools have also been evaluated for assessing additional postoperative adverse events, alongside poor pain management, including the 4 A’s test (4AT) for postoperative delirium[27].
Table 2 shows the ‘Pain assessment in advanced dementia’ (PAINAD) scale[28].
Recently, the concept of administering sufficient analgesia to promote drinking, eating and mobilising — referred to by the mnemonic ‘DrEaMing’ has gained prominence. DrEaMing is now one of the five priorities of the Perioperative Quality Improvement Programme[6]. It is now recommended not to treat according to pain intensity and not to attempt to remove all pain, but to promote DrEaMing[6].
Pharmacological management
A multimodal approach is recommended to manage postoperative pain and forms an important part of Enhanced Recovery after Surgery (ERAS) pathways[19,29]. ERAS pathways aim for shorter hospital stays and less postoperative complications and typically include early mobilisation and early food intake[19].
Multimodal analgesia uses a combination of different drug classes and analgesic techniques[30]. Using analgesics with different mechanisms of action combined to produce synergistic effects is associated with a reduction in side effects, owing to the lower doses of analgesics being used and differences in their side effect profiles[31]. Intra-operatively, opioid-sparing analgesic techniques, such as peripheral nerve blocks, and evidence-based, procedure-specific analgesic techniques are used where possible and form the main components of multimodal analgesia[16,30]. The recent expansion of the types and number of nerve block techniques for different types of surgery poses an increasing challenge for anaesthetists and appropriate training is essential to ensure adequate analgesia and limitation of adverse effects[30]. Further discussion on the utilisation of multimodal analgesia in different procedures is outside the scope of this article.
Opioids
Postoperative IV opioids and non-opioids may be used under the recommendation of the surgeon and anaesthetist (e.g. morphine, oxycodone, fentanyl and bupivacaine) following major surgical procedures[6]. Patient-controlled analgesia (PCA) and epidurals are alternative forms of administration. For more details, see ‘Further reading‘.
Opioids (e.g. morphine and oxycodone) should only be offered when immediate postoperative pain is expected to be moderate to severe[19]. The general expectation is that postoperative pain should resolve during the healing process. Modified release opioids should be avoided for postoperative pain management, as patches or modified/controlled release preparations do not allow for the rapid dose adjustment during recovery that may be required, therefore immediate release opioids are preferred when simple analgesics are insufficient[2]. Oral opioids (e.g. codeine and morphine) should be given as soon as the patient can eat and drink, with the dose adjusted to help the patient achieve functional recovery (such as coughing and mobilising), with oral morphine solution commonly used on an in-patient basis[6,19]. At discharge, the prescribing of opioids should be minimised and the use of non-opioids encouraged[32]. When prescribing or screening prescriptions for any opioid, the need for laxatives or anti-emetics should be determined through the use of observational tools, such as the Bristol Stool Form Scale, as well as nausea and vomiting observations[33,34].
Ketamine
If a patient’s pain is expected to be moderate to severe and an IV opioid alone does not provide adequate pain relief or a patient has opioid sensitivity, ketamine may be considered. Ketamine may be considered either during or immediately after surgery as a single IV dose to supplement other pain relief[19]. Ketamine is thought to reduce the likelihood of transition to PPSP[35]. In 2018, Brinck et al. reviewed 130 studies and found postoperative opioid consumption and pain intensity over 48 hours were lower with perioperative IV ketamine[36].
The inclusion of ketamine may be effective in patients with escalating opioid requirements but the benefits of its use must be balanced with dose-dependent adverse effects, including hypersalivation, nausea and vomiting and psychotomimetic effects[1].
Paracetamol
Results of a systematic review published by Maund et al. in 2011 showed a statistically significant reduction in morphine consumption when paracetamol or a non-steroidal anti-inflammatory drug (NSAID) is given after surgery[37]. Paracetamol should be prescribed irrespective of pain severity, in the absence of contraindications, such as hypersensitivity to paracetamol[19,38]. IV paracetamol should only be used when the oral route is not available. A reduced dose is advised for patients with a low body weight (less than 50kg) or risk factors for hepatotoxicity[38]. Paracetamol is available to buy OTC, allowing patients to self-medicate after discharge according to their discharge plan for management of pain. Although patients’ own supply of OTC simple analgesia is recommended, adequate provision for those unable to access this, such as people living in nursing homes or secure environments, and those without a fixed abode, needs to be ensured.
Non-steroidal anti-inflammatory drugs
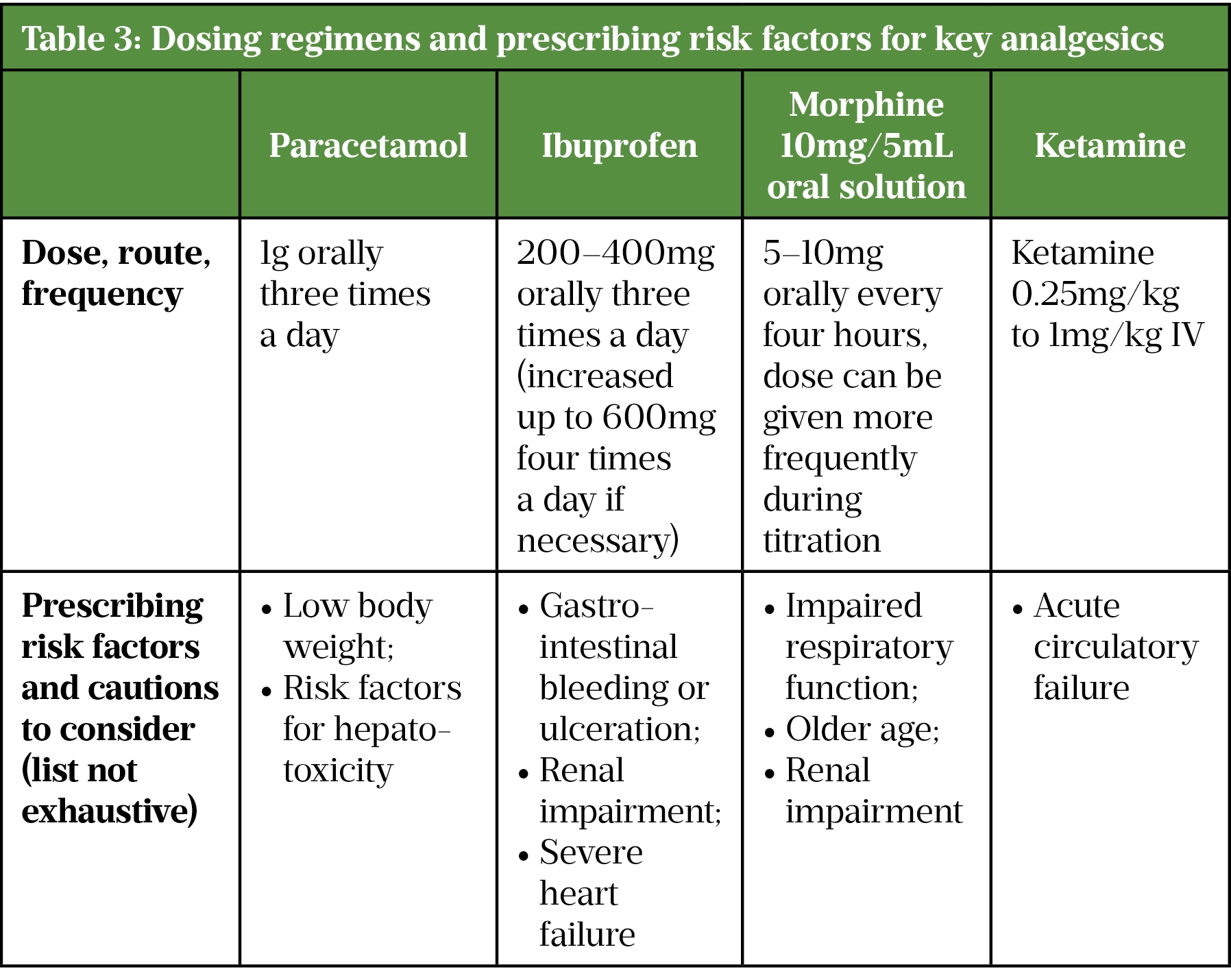
Non-steroidal anti-inflammatory drugs (NSAIDs) should be offered to manage postoperative pain irrespective of severity, in the absence of contraindications such as renal impairment (see Table 3)[19,38]. The lowest effective dose of an NSAID should be prescribed for the shortest period of time to control symptoms, determined by the patient’s pain scores, ability to mobilise and number of doses refused[39]. All NSAID use can be associated with a small increased risk of thrombotic events independent of baseline cardiovascular risk factors or duration of NSAID use; however, the greatest risk may be in those receiving high doses long term (i.e. more than three times a week for more than three months)[39].
Ibuprofen has fewer side effects than other non-selective NSAIDs, but its anti-inflammatory properties are weaker[38]. Cyclo-oxygenase-2 (COX-2) inhibitors, diclofenac (150mg daily) and ibuprofen (2.4g daily) are associated with an increased risk of thrombotic events and should be avoided[38]. Low doses of ibuprofen (1.2g daily or less) have not been associated with an increased risk of myocardial infarction[38]. However, NSAIDs are not recommended in patients who have had surgery for a hip fracture, owing to the risk of a secondary hip fracture[40,41]. NSAIDs are thought to prevent fragility fractures through improvements in bone mineral density; however, observational studies have reported a link between NSAIDs and secondary hip fractures in patients who have had surgery for a hip fracture[41].
NSAIDs are available for use in a number of different routes, including parental, oral and rectal[38]. IV NSAIDs are also not routinely recommended unless the patient is unable to take oral medication[19]. Oral ibuprofen can be offered for postoperative pain as it remains an effective analgesic and in the same way as paracetamol it is available to buy OTC if required, allowing short-term self-medication on discharge. Pharmacists should ensure that a patient’s renal function and any previous sensitivity to NSAIDs is investigated before prescribing or screening the prescription.
Gabapentin
Gabapentin can be used to treat peripheral neuropathic pain and may have a role in supplementing other forms of pain relief, with limited evidence of benefits in procedures ranging from hysteroscopies to pneumonectomies[19]. There is inconclusive evidence for its use, with some studies suggesting there are minimal opioid sparing effects and an increased risk of adverse events[1,19]. Adverse effects of using gabapentin include anxiety, dizziness and double vision, known as diplopia[38].
In 2019, gabapentin was reclassified as schedule 3 under the Misuse of Drugs Regulations 2001, the MHRA published guidance on prescribing gabapentin owing to the growing concern of abuse[42]. When prescribing gabapentin, patients should be evaluated carefully for a history of drug abuse (for example, through the use of screening tools) and dependence before prescribing[42]. Patients on gabapentin should be observed for possible signs of abuse (e.g. extreme fatigue, often described as ‘zombie-like’ effects) and dependence and ensure that patients are aware of the risk of potentially fatal interactions with other medicines that cause central nervous system and respiratory depression, particularly opioids and alcohol[42,43].
Non-pharmacological measures
Established adjunctive methods for the management of postoperative pain include physiotherapy[44]. Other non-pharmacological measures have been explored and have the advantage of being low cost, carrying low risk of dependency and being relatively easy to implement[1,45]. There is currently insufficient evidence to recommend a specific type of non-pharmacological measure for each type of surgery; however, various measures have been gaining prominence in pain management and feature predominantly in the NICE guidance for the management of chronic primary pain[46]. Various strategies have been implemented post-operatively and can be divided into four main groups:
- Physical application — transcutaneous electrical nerve stimulation (TENS), acupuncture and heat or cold packs;
- Physical activity — light or moderate exercise, deep breathing;
- Spiritual and psychological — meditation, praying, visualisation, cognitive behavioural therapy (CBT);
- Distractions — conversations, music, television[1,45].
Effective patient education and a multidisciplinary approach is also thought to improve postoperative pain management[1,47].
Pharmacy teams and pain
Ensuring appropriate peri-operative opioid use is an important responsibility for all healthcare professionals, including pharmacy professionals. The Royal College of Anaesthetists (RCoA) ‘Guidelines for the provision of anaesthesia services for inpatient pain management 2022’ highlights the role of pharmacists in all stages of the peri-operative pain management process[48]. Figure 3 summarises best practice guidelines for optimal PSP management, with the potential involvement of pharmacy teams highlighted throughout[6,49].

NSAIDs: non-steroidal anti-inflammatory drugs, P: pharmacist, PT: pharmacy technician, WHO: World Health Organization
At a ward-level, respiratory rate and sedation for opioids should be routinely monitored, with side effects, including opioid-induced constipation, appropriately managed to ensure their effective use. It is good practice to ensure naloxone is prescribed alongside opioids for emergency use[50]. Care should be taken with naloxone use in patients who receive longer-term opioid treatment or who are physically dependent on opioids[50]. Two dose ranges of naloxone are recommended[38]. One is a high-dose regimen for rapid titration necessary to reduce potentially life-threatening effects and the second a low-dose regimen when there is a risk of acute withdrawal, or when a continued therapeutic effect is required[38].
Pharmacists screening these prescriptions should be aware of this difference. Inappropriate naloxone use can cause a rapid reversal of the physiological effects for pain control, leading to intense pain and distress (requiring reestablishment of opioid analgesia) and an increase in sympathetic nervous stimulation and cytokine release. This precipitates an acute withdrawal syndrome[50]. Hypertension, cardiac arrhythmias, pulmonary oedema and cardiac arrest may result from inappropriate doses of naloxone being used[50].
Postoperatively, the requirement for opioids requires regular review. If a patient requires frequent doses, there should be a discussion between the patient and prescriber to establish a plan for reducing opioid use prior to discharge. When screening prescriptions for opioids, as well as reviewing renal function, pharmacists should be aware of other patient specific factors, such as variable metabolism of codeine (see ‘Further reading‘ for more information) and the potential interactions and side effects associated with tramadol[38].
Pharmacists should ensure that when screening discharge prescriptions, the amount and type of analgesia prescribed on discharge is appropriate. The dose, frequency, previous medication history and the ongoing requirement should be considered.
In 2015, Calcaterra et al. published their retrospective study on opioid prescribing on discharge from hospital, results of which found there was an increased risk of future chronic opioid use in those discharged with opioids from hospital[51]. Therefore, the discharge summary should clearly state the amount to supply and plan for review after discharge, either by primary or secondary care. It is preferable to prescribe opioid and non-opioid analgesics separately to allow for dose changes of individual analgesics; for example, codeine and paracetamol instead of co-codamol. Patients should also be encouraged to keep a record of analgesics taken, as research has shown that this results in better pain control[48]. In 2018, Brat et al. found that duration of the prescription rather than dosage is more strongly associated with ultimate misuse in the early post-surgical period[52]. Therefore, where practical, no more than seven days of opioids should be prescribed[48].
Summary
The prevention and management of PSP is an important responsibility for all involved in peri-operative care[1,2]. Best practice points for pharmacy teams include[6,49]:
- Optimisation, psychological preparation and expectation management;
- Referral to pain specialists for complex cases;
- Postoperative pain assessment and pain management strategies to promote return of normal function (DrEaMing);
- Patient education regarding self-administration of opioids;
- Adequate transfer of care, including full documentation in the patient’s hospital discharge letter;
- Postoperative opioids must not be added to a “repeat” — if extended use is needed acute prescriptions should be issued, with a review prior to each issue.
From screening for chronic pain and opioid use preoperatively, to supporting postoperative opioid management, each pharmacy professional can play a significant role in reducing PPSP occurrence[6,49].
Further reading
Schaible HG & Richter F. ‘Pathophysiology of pain‘, Langenbeck’s Archives of Surgery, 2004
Hindmarsh J, Kai Au Y & Jonathan Pickard J. ‘How codeine metabolism affects its clinical use‘, The Pharmaceutical Journal online, 2021
Rahman M & Beattie J. ‘Managing postoperative pain through giving patients control‘, The Pharmaceutical Journal online, 2005
Robinson J. ‘Opioids: what can be done about long-term prescribing‘, The Pharmaceutical Journal online, 2022
Connelly D. ‘In figures: the rise in long-term opioid use‘, The Pharmaceutical Journal online, 2022
Moore R, Wiffen PJ, Sheena Derry S et al. ‘Non‐prescription (OTC) oral analgesics for acute pain — an overview of Cochrane reviews‘, Cochrane Database of Systematic Reviews, 2015
- 1Small C, Laycock H. Acute postoperative pain management. British Journal of Surgery. 2020;107:e70–80. doi:10.1002/bjs.11477
- 2Levy N, Mills P, Rockett M. Post-surgical pain management: time for a paradigm shift. British Journal of Anaesthesia. 2019;123:e182–6. doi:10.1016/j.bja.2019.05.031
- 3Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain 1979;6:249.https://www.ncbi.nlm.nih.gov/pubmed/460932
- 4Gupta A, Kaur K, Sharma S, et al. Clinical aspects of acute post-operative pain management & its assessment. J Adv Pharm Technol Res 2010;1:97–108.https://www.ncbi.nlm.nih.gov/pubmed/22247838
- 5Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. JPR. 2017;Volume 10:2287–98. doi:10.2147/jpr.s144066
- 6Srivastava D, Hill S, Carty S, et al. Surgery and opioids: evidence-based expert consensus guidelines on the perioperative use of opioids in the United Kingdom. British Journal of Anaesthesia. 2021;126:1208–16. doi:10.1016/j.bja.2021.02.030
- 7Soffin EM, Lee BH, Kumar KK, et al. The prescription opioid crisis: role of the anaesthesiologist in reducing opioid use and misuse. British Journal of Anaesthesia. 2019;122:e198–208. doi:10.1016/j.bja.2018.11.019
- 8Current UK data on opioid misuse. Faculty of Pain Medicine of the Royal College of Aneasthetists. https://www.fpm.ac.uk/opioids-aware-clinical-use-opioids/current-uk-data-opioid-misuse (accessed Sep 2022).
- 9Gomes T, Tadrous M, Mamdani MM, et al. The Burden of Opioid-Related Mortality in the United States. JAMA Netw Open. 2018;1:e180217. doi:10.1001/jamanetworkopen.2018.0217
- 10van Helmond N, Olesen SS, Wilder-Smith OH, et al. Predicting Persistent Pain After Surgery. Anesthesia & Analgesia. 2018;127:1264–7. doi:10.1213/ane.0000000000003318
- 11Bui T, Fitzpatrick B, Forrester T, et al. Standard of practice in surgery and perioperative medicine for pharmacy services. Pharmacy Practice and Res. 2022;52:139–58. doi:10.1002/jppr.1805
- 12Levy N, Quinlan J, El‐Boghdadly K, et al. An international multidisciplinary consensus statement on the prevention of opioid‐related harm in adult surgical patients. Anaesthesia. 2020;76:520–36. doi:10.1111/anae.15262
- 13Velayudhan A, Bellingham G, Morley-Forster P. Opioid-induced hyperalgesia. Continuing Education in Anaesthesia Critical Care & Pain. 2014;14:125–9. doi:10.1093/bjaceaccp/mkt045
- 14Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2015;25:733–7. doi:10.1002/pds.3945
- 15Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia—when is enough too much? A review of opioid-induced tolerance and hyperalgesia. The Lancet. 2019;393:1558–68. doi:10.1016/s0140-6736(19)30430-1
- 16Surgery and Opioids: Best Practice Guidelines 2021. Faculty of Pain Medicine. 2021.https://fpm.ac.uk/sites/fpm/files/documents/2021-03/surgery-and-opioids-2021_4.pdf (accessed Sep 2022).
- 17Powell R, Scott NW, Manyande A, et al. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database of Systematic Reviews. 2016;2016. doi:10.1002/14651858.cd008646.pub2
- 18Baldo BA. Opioid analgesic drugs and serotonin toxicity (syndrome): mechanisms, animal models, and links to clinical effects. Arch Toxicol. 2018;92:2457–73. doi:10.1007/s00204-018-2244-6
- 19Perioperative care in adults. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ng180 (accessed Sep 2022).
- 20Inacio MCS, Hansen C, Pratt NL, et al. Risk factors for persistent and new chronic opioid use in patients undergoing total hip arthroplasty: a retrospective cohort study. BMJ Open. 2016;6:e010664. doi:10.1136/bmjopen-2015-010664
- 21Winning abstracts from the National Acute Pain Symposium 2019. British Journal of Pain. 2019;:204946371989579. doi:10.1177/2049463719895793
- 22Genord C, Frost T, Eid D. Opioid exit plan: A pharmacist’s role in managing acute postoperative pain. Journal of the American Pharmacists Association. 2017;57:S92–8. doi:10.1016/j.japh.2017.01.016
- 23Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. British Journal of Anaesthesia. 2008;101:17–24. doi:10.1093/bja/aen103
- 24Chiu LYL, Sun T, Ree R, et al. The evaluation of smartphone versions of the visual analogue scale and numeric rating scale as postoperative pain assessment tools: a prospective randomized trial. Can J Anesth/J Can Anesth. 2019;66:706–15. doi:10.1007/s12630-019-01324-9
- 25Core Standards for Pain Management Services in the UK 2nd Edition. Faculty of Pain Medicine. 2021.https://fpm.ac.uk/sites/fpm/files/documents/2021-02/CSPMS-version-open-consultation.pdf (accessed Sep 2022).
- 26Schofield P. The Assessment of Pain in Older People: UK National Guidelines. Age and Ageing. 2018;47:i1–22. doi:10.1093/ageing/afx192
- 27Saller T, MacLullich AMJ, Schäfer ST, et al. Screening for delirium after surgery: validation of the 4 A’s test (4AT) in the post‐anaesthesia care unit. Anaesthesia. 2019;74:1260–6. doi:10.1111/anae.14682
- 28Tyler KR, Hullick C, Newton BA, et al. <scp>Emergency department</scp>pain management in older patients. Emergency Medicine Australasia. 2020;32:840–6. doi:10.1111/1742-6723.13562
- 29Gelman D, Gelmanas A, Urbanaitė D, et al. Role of Multimodal Analgesia in the Evolving Enhanced Recovery after Surgery Pathways. Medicina. 2018;54:20. doi:10.3390/medicina54020020
- 30Shim JH. Multimodal analgesia or balanced analgesia: the better choice? Korean J Anesthesiol. 2020;73:361–2. doi:10.4097/kja.20505
- 31Kehlet H, Dahl JB. The Value of ???Multimodal??? or ???Balanced Analgesia??? in Postoperative Pain Treatment. Anesthesia & Analgesia. 1993;77:1048???1056. doi:10.1213/00000539-199311000-00030
- 32Clarke HA, Manoo V, Pearsall EA, et al. Consensus Statement for the Prescription of Pain Medication at Discharge after Elective Adult Surgery. Canadian Journal of Pain. 2020;4:67–85. doi:10.1080/24740527.2020.1724775
- 33Chumpitazi BP, Self MM, Czyzewski DI, et al. Bristol Stool Form Scale reliability and agreement decreases when determining Rome III stool form designations. Neurogastroenterol. Motil. 2015;28:443–8. doi:10.1111/nmo.12738
- 34Carr E, Bendayan R, Bean D, et al. Evaluation and Improvement of the National Early Warning Score (NEWS2) for COVID-19: a multi-hospital study. 2020. doi:10.1101/2020.04.24.20078006
- 35Chaparro LE, Smith SA, Moore RA, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database of Systematic Reviews. 2013;2021. doi:10.1002/14651858.cd008307.pub2
- 36Brinck E, Tiippana E, Heesen M, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. CDSR. 2018. doi:10.1002/14651858.cd012033.pub4
- 37Maund E, McDaid C, Rice S, et al. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. British Journal of Anaesthesia. 2011;106:292–7. doi:10.1093/bja/aeq406
- 38Joint Formulary Committee. British National Formulary (BNF). 83rd ed. BMJ Group and Pharmaceutical Press. British National Formulary. 2022.https://bnf.nice.org.uk (accessed Sep 2022).
- 39Cox-2 selective inhibitors and non-steroidal anti-inflammatory drugs’ (NSAIDs): Cardiovascular safety. Medicines and Healthcare products Regulatory Agency. 2015.https://www.gov.uk/government/publications/cox-2-selective-inhibitors-and-non-steroidal-anti-inflammatory-drugs-nsaids-cardiovascular-safety (accessed Sep 2022).
- 40National Institute for Health and Care Excellence. Hip Fracture: Management. 2011.https://www.nice.org.uk/guidance/cg124/chapter/recommendations (accessed Sep 2022).
- 41Chuang P-Y, Shen S-H, Yang T-Y, et al. Non-steroidal anti-inflammatory drugs and the risk of a second hip fracture: a propensity-score matching study. BMC Musculoskelet Disord. 2016;17. doi:10.1186/s12891-016-1047-2
- 42Pregabalin (Lyrica), gabapentin (Neurontin) and risk of abuse and dependence: new scheduling requirements from 1 April. Medicines and Healthcare products Regulatory Agency. 2019.https://www.gov.uk/drug-safety-update/pregabalin-lyrica-gabapentin-neurontin-and-risk-of-abuse-and-dependence-new-scheduling-requirements-from-1-april (accessed Sep 2022).
- 43Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62:406–7. doi:10.3399/bjgp12x653516
- 44Aldhuhoori FZ, Walton L, Bairapareddy KC, et al. Physiotherapy Practice for Management of Patients Undergoing Upper Abdominal Surgery in United Arab Emirates – A National Survey. JMDH. 2021;Volume 14:2513–26. doi:10.2147/jmdh.s328528
- 45Komann M, Weinmann C, Schwenkglenks M, et al. Non-Pharmacological Methods and Post-Operative Pain Relief: An Observational Study. Anesth Pain Med. 2019;In Press. doi:10.5812/aapm.84674
- 46Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ng193 (accessed Sep 2022).
- 47Jones DB, Abu-Nuwar MRA, Ku CM, et al. Less pain and earlier discharge after implementation of a multidisciplinary enhanced recovery after surgery (ERAS) protocol for laparoscopic sleeve gastrectomy. Surg Endosc. 2020;34:5574–82. doi:10.1007/s00464-019-07358-w
- 48Guidelines for the Provision of Anaesthetic Services. Royal College of Anaesthetists. https://rcoa.ac.uk/safety-standards-quality/guidance-resources/guidelines-provision-anaesthetic-services (accessed Sep 2022).
- 49Levy N, Mills P, Mythen M. Is the pursuit of DREAMing (drinking, eating and mobilising) the ultimate goal of anaesthesia? Anaesthesia. 2016;71:1008–12. doi:10.1111/anae.13495
- 50Patient safety alert – risk of distress and death from inappropriate doses of naloxone in patients on long-term opioid or opiate treatment. NHS England. 2014.https://www.england.nhs.uk/2014/11/psa-naloxone/ (accessed Sep 2022).
- 51Calcaterra SL, Yamashita TE, Min S-J, et al. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J GEN INTERN MED. 2015;31:478–85. doi:10.1007/s11606-015-3539-4
- 52Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;:j5790. doi:10.1136/bmj.j5790


