Science Photo Library
After reading this article, you should be able to:
- Appreciate that codeine is a prodrug, which is metabolised into morphine;
- Understand that codeine’s conversion into morphine shows significant variability and is influenced by many factors;
- Comprehend the clinical consequences of such variable metabolism;
- Monitor patients taking codeine, and consider when alternative opioids may be appropriate.
Codeine is an established, familiar and widely used analgesic. It is considered a weak opioid, with a potency around one tenth that of morphine (i.e. 60mg of codeine is equivalent to around 6mg of morphine)[1]. Codeine is administered in doses of 15–60mg up to four times per day (maximum of 240mg in 24 hours) and confers beneficial analgesic, antitussive and antidiarrhoeal effects[2,3].
Despite its utility and ubiquity, codeine actually has little or no analgesic activity until it is metabolised into morphine[4,5]. Consequently, codeine can be regarded as a prodrug (a compound that is pharmacologically inactive until it is metabolised into an active form by the human body)[6].
The extent of this metabolism varies however: individuals with differing CYP2D6 enzyme activity may derive differing effects (and associated adverse effects) from the same dose of codeine. Additional limitations include the potential for drug–drug interactions, and a so-called ‘ceiling effect’ that adversely tips the risk–benefit scale at supratherapeutic doses[2,3,6]. To appreciate the wide-ranging clinical significance of these factors fully, the metabolism of codeine must first be understood.
Metabolism
Codeine is primarily metabolised by two different pathways (see Figure)[2]. In most people, around 80% of codeine is conjugated to form codeine-6-glucuronide, which may have weak analgesic activity[2,7]. However, typically less than 10% of codeine undergoes CYP2D6-mediated O-demethylation to the potent analgesic, morphine[5].
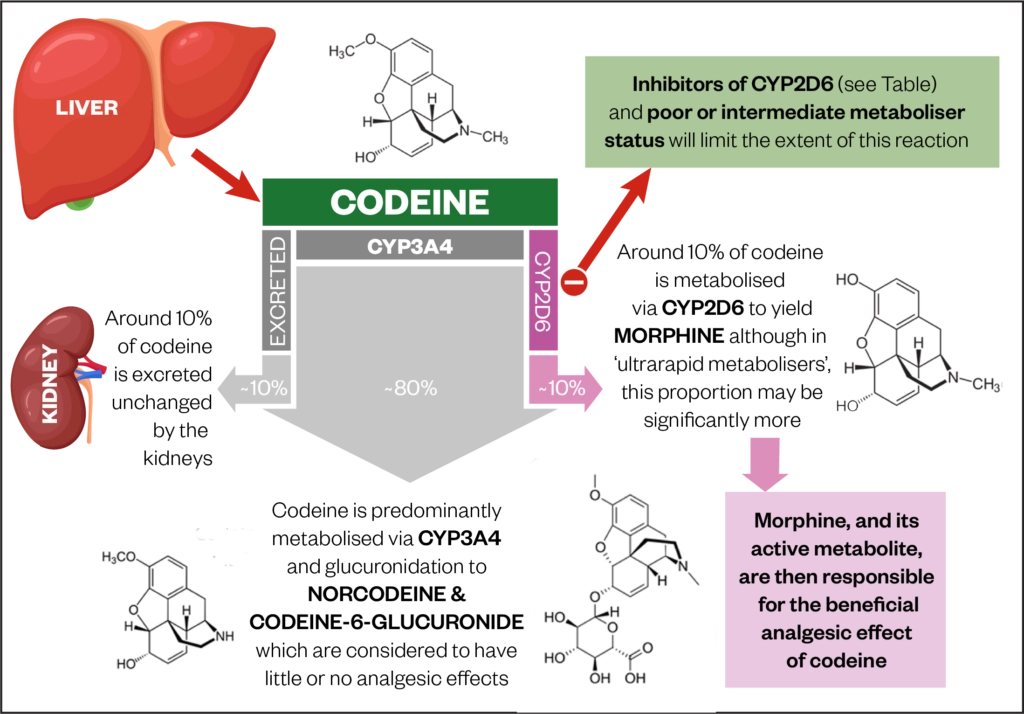
Source: J Pickard and J Hindmarsh, adapted from Gasche et al.[8]
The CYP2D6 enzyme belongs to the CYP450 super-family of enzymes, which are responsible for the metabolism of many drugs and endogenous compounds[9].
These enzymes exist throughout the body, but are most prevalent in the liver. The expression and function of these enzymes can be altered by genetic variation, with large inter-ethnic differences being observed[10]. For example, clinically significant alterations in CYP2D6 functionality are known to affect Caucasians (5–10%) and Africans (0–34%) more than Asians (≤1%)[10–12].
Such variations can significantly affect how an individual responds to a drug[11]. This can be said to be true of codeine[9,10]. The extent of that conversion can vary from none to potentially too much, meaning that, for some people, codeine may not be the right choice of analgesic[8,13–15].
Identifying CYP2D6 metabolism status
Although pharmacogenetic testing can determine a patient’s genotype and CYP2D6 functionality, in practice, such testing has not been shown to be cost effective and is therefore not routinely employed prior to initiating drugs such as codeine[10–12]. Nevertheless, as differing CYP2D6 metaboliser statuses can have clinically observable consequences for patients, it remains important to understand how the metabolism of codeine can be affected by these genetic differences. CYP2D6 statuses are classified as poor, intermediate, extensive or ultra-rapid (see Box)[10,13].
Box: CYP2D6 statuses and the associated analgesic benefit versus potential adverse effects
- Poor metabolisers (5–10% of individuals) lack functional enzymes and will thus derive little to no analgesic benefit from codeine, owing to an inability to convert it into its active form, morphine[10,13]. Poor metabolisers still experience similar rates of adverse effects (such as sedation, dizziness, euphoria, blurred vision and dry mouth) from codeine when compared with the general population[14,15]. This subset of individuals may experience undesirable effects, without any clinical analgesic benefit.
- Intermediate metabolisers (2–11% of individuals) may express enzymes with decreased function or have a combination of functioning and non-functioning enzymes[10,13]. Consequently, drug metabolism may be reduced, and such individuals may also derive little benefit from codeine[10,13].
- Most individuals (77–92%) are extensive metabolisers. They have normal enzyme activity, and will metabolise around 10% of codeine to morphine, deriving analgesic benefit[13].
- A rarer, but not-to-be-overlooked subset (1–2% of individuals) are considered ultra-rapid metabolisers. Ultra-rapid metabolisers have increased enzymatic activity, which leads to them metabolising a greater proportion of codeine into morphine than the general population[10,13]. This is potentially very hazardous: Gasche et al. described a case of life-threatening opioid toxicity in a man aged 62 years who had received a low dose of codeine (75mg per day) for management of cough[8]. The patient was later identified as being a CYP2D6 ultra-rapid metaboliser. Unfortunately, similar cases have been reported in children, some of which have resulted in fatalities[16]. Consequently, codeine for management of pain, cough and diarrhoea is contraindicated in patients aged under 12 years[17,18].
Hence, up to nearly a quarter of individuals may show a response to codeine that ranges from inefficacy — but still with adverse effects — to potentially life-threatening toxicity[11].
In practice, the CYP2D6 metabolism status of patients can be determined through clinical observation. Poor metabolisers may experience limited to no therapeutic response to codeine (also see drug–drug interactions below), while those displaying signs of opioid toxicity — such as respiratory depression, myoclonic twitches, confusion and hallucinations — may be ultra-rapid metabolisers[8,13,17]. In both cases, the most appropriate course of action may be to discontinue codeine and decide, along with the patient and prescriber, on an alternative analgesic. For example, pharmacists can liaise with prescribers and suggest starting low-dose morphine (10mg modified-release morphine sulphate twice daily) in place of codeine, if appropriate. If the patient is thought to be a poor metaboliser, a ‘wash out’ period does not need to be observed, but if toxicity to codeine is suspected, prescribers should wait for signs of toxicity to abate as the drug washes out before prescribing an alternative. In the latter scenario, specialist input is advised[1,13,19].
Metabolism status aside, the co-administration of drugs that alter the function of CYP2D6 must also be considered as an important determinant of the variable efficacy of codeine.
Drug–drug interactions
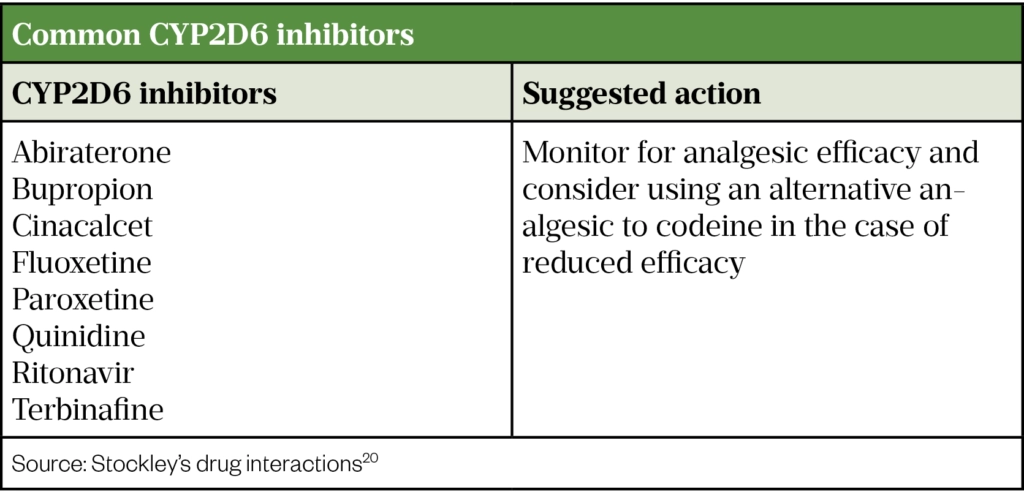
Medications that inhibit CYP2D6 are predicted to diminish, or abolish, the effect of codeine by preventing its metabolism into morphine. These are outlined in the Table[15,20]. Therefore, regardless of CYP2D6 status, inefficacy of codeine may occur as a result of a drug–drug interaction. Patients reporting limited to no therapeutic reponse from codeine should also have their medications reviewed for the presence of CYP2D6 inhibitors (see Table). For people taking CYP2D6 inhibitors, conversion to an alternate opioid which does not rely on CYP2D6 metabolism (e.g. morphine) should be considered[20,21].
The ‘ceiling’ effect
When used for the management of pain, codeine is generally considered to have a ‘ceiling’ effect. Although this is not an absolute ceiling, this term describes a point where the analgesic benefit of further dose escalation is often outweighed by the increasing burden of adverse effects, thus limiting dose escalation beyond a certain point[21–26]. Doses of codeine should not exceed the licensed maximum of 240mg in 24 hours (in divided doses)[3]. This maximum has been derived in part from studies indicating that dose escalations beyond this point cause an increase in adverse effects —including sedation, dizziness, nausea and vomiting — with limited additional analgesic efficacy[21–26]. In practice, the ceiling effect is circumvented by prescribing within the licensed therapeutic window.
As doses can vary between 15–60mg up to 4 times per day, it is important that patients are counselled on the strength and number of tablets they need to take and that a minimum of 6 hours should pass before taking the next dose[2,3].
Monitoring
When reviewing patients, the analgesic benefit of codeine should be determined by asking patients to score their pain out of ten (where ten represents the worst pain imaginable) and assess for improvement post initiation of codeine. Additionally, functional and behavioural outcomes which may also infer analgesic response should be evaluated (e.g. if the patient reports increased physical activity, owing to a reduction in pain).
Patients should be closely monitored for adverse effects, therapeutic response and the balance between the two. Many adverse effects can be dose limiting (e.g. confusion, dizziness, hallucinations, nausea and vomiting)[1]. Constipation and dry mouth are class effects of all opioids, to which tolerance does not develop and should be managed accordingly depending on severity[1].
Patients should be informed that codeine can cause drowsiness, which may affect the performance of skilled tasks, such as driving[1]. Such sedative effects can also be enhanced by alcohol and other sedative medications (e.g. benzodiazepines)[20,21]. Patients should not drive at the start of codeine therapy, or following a dose titration[27,28]. In both cases, it may take five days, or more, for the drowsiness to resolve and patients should only drive if they feel 100% safe to do so[29].
Concomitant use of codeine and other opioids (such as tramadol, morphine, oxycodone, hydromorphone or fentanyl) or opioid-containing preparations (such as over-the-counter co-codamol) can increase the risk of opioid toxicity. Pharmacists should ensure patients are made aware that codeine is an opioid and that use alongside other opioids should be avoided[1,20].
It is important that patients and carers are counselled on how to recognise the signs and symptoms of opioid toxicity (including reduced consciousness, somnolence, respiratory depression, ‘pin-point’ pupils, nausea and vomiting). In such an event, urgent medical attention should be sought. The risk of respiratory depression is also increased when opioids are used alongside benzodiazepines and/or gabapentinoids[3,15,30,31].
Alternative solutions
Codeine’s latent and unpredictable spectrum of efficacy, susceptibility for interaction with common medications and a ‘ceiling’ limiting dose escalations must be kept in mind by prescribers. Use of alternative opioids with superior efficacy and safety should be considered; particularly for cancer pain and in paediatrics and post-operative settings[27,28,30–32].
Getting around these issues can be simple, however: for cancer pain, evidence now advocates ‘skipping the prodrug’ and substituting codeine with low-dose morphine, which was shown to be superior to codeine, with patients achieving greater and more rapid onset of pain reduction with morphine than with codeine[32]. Some studies in neurosurgery have also shown greater patient satisfaction with morphine compared with codeine[33]. In paediatrics and palliative care, it has become commonplace to avoid ‘weak’ opioids in favour of initiating low doses of ‘strong’ opioids, such as morphine[29,34]. Oral morphine is also commonly used post-operatively in preference to codeine[35].
Overall, codeine remains established, accessible and — in the majority — effective as an analgesic, but it is important to remain mindful of its limitations. Garnering the most beneficial effects through its metabolism to morphine, prescribers should consider carefully when alternatives to codeine as a weak opioid may be favourable, more predictable or safer.
References
- 1Pickard J, McDonald E, Hindmarsh J. Opioid use in palliative care: selection, initiation and optimisation. The Pharmaceutical Journal 2020;305. doi:10.1211/PJ.2020.20208535
- 2Compound summary: Codeine. PubChem. 2021.https://pubchem.ncbi.nlm.nih.gov/compound/Codeine (accessed Apr 2021).
- 3British National Formulary (online). Joint Formulary Committee: London: BMJ Group and Pharmaceutical Press. 2021.http://www.medicinescomplete.com (accessed Apr 2021).
- 4Vree T, Verwey-van W. Pharmacokinetics and metabolism of codeine in humans. Biopharm Drug Dispos 1992;13:445–60. doi:10.1002/bdd.2510130607
- 5Dayer P, Desmeules J, Leemann T, et al. Bioactivation of the narcotic drug codeine in human liver is mediated by the polymorphic monooxygenase catalyzing debrisoquine 4-hydroxylation (cytochrome P-450 dbl/bufI). Biochem Biophys Res Commun 1988;152:411–6. doi:10.1016/s0006-291x(88)80729-0
- 6Anderson B. Is it farewell to codeine? Arch Dis Child 2013;98:986–8. doi:10.1136/archdischild-2013-304974
- 7Vree T, van D, Koopman-Kimenai P. Codeine analgesia is due to codeine-6-glucuronide, not morphine. Int J Clin Pract 2000;54:395–8.https://www.ncbi.nlm.nih.gov/pubmed/11092114
- 8Gasche Y, Daali Y, Fathi M, et al. Codeine Intoxication Associated with Ultrarapid CYP2D6 Metabolism. N Engl J Med 2004;351:2827–31. doi:10.1056/nejmoa041888
- 9Haufroid V, Hantson P. CYP2D6 genetic polymorphisms and their relevance for poisoning due to amfetamines, opioid analgesics and antidepressants. Clin Toxicol (Phila) 2015;53:501–10. doi:10.3109/15563650.2015.1049355
- 10Twycross G, Wilcock A, Howard P, et al., editors. Variability in response to drugs. In: Palliative Care Formulary. London: : Pharmaceutical Press 2020. N/A.
- 11Wu AHB. Drug metabolizing enzyme activities versus genetic variances for drug of clinical pharmacogenomic relevance. Clin Proteom 2011;8. doi:10.1186/1559-0275-8-12
- 12Kimmel SE, French B, Kasner SE, et al. A Pharmacogenetic versus a Clinical Algorithm for Warfarin Dosing. N Engl J Med 2013;369:2283–93. doi:10.1056/nejmoa1310669
- 13Pratt V, Scott S, Pirmohamed M, et al. gtrbook. Published Online First: 30 March 2021.http://www.ncbi.nlm.nih.gov/books/NBK100662/
- 14Eckhardt K, Li S, Ammon S, et al. Same incidence of adverse drug events after codeine administration irrespective of the genetically determined differences in morphine formation. Pain 1998;76:27–33. doi:10.1016/s0304-3959(98)00021-9
- 15Twycross G, Wilcock A, Howard P, et al., editors. Codeine phosphate. In: Palliative Care Formulary. London: : Pharmaceutical Press 2020. N/A.
- 16Ciszkowski C, Madadi P, Phillips M, et al. Codeine, ultrarapid-metabolism genotype, and postoperative death. N Engl J Med 2009;361:827–8. doi:10.1056/NEJMc0904266
- 17Codeine not to be used in children below 12 years for cough and cold. European Medicines Agency. 2015.https://www.ema.europa.eu/en/news/codeine-not-be-used-children-below-12-years-cough-cold (accessed Apr 2021).
- 18Mitchell RB, Archer SM, Ishman SL, et al. Clinical Practice Guideline: Tonsillectomy in Children (Update). Otolaryngol Head Neck Surg 2019;160:S1–42. doi:10.1177/0194599818801757
- 19Twycross G, Wilcock A, Howard P, et al., editors. Quick clinical guide: Reversal of opioid-induced respiratory depression. In: Palliative Care Formulary. London: : Pharmaceutical Press 2020. N/A.
- 20Preston C. Stockley’s drug interactions. London: : Pharmaceutical Press 2015.
- 21Sindrup SH, Br??sen K. The pharmacogenetics of codeine hypoalgesia. Pharmacogenetics 1995;5:335–46. doi:10.1097/00008571-199512000-00001
- 22LLOYD-THOMAS AR. Pain management in paediatric patients. British Journal of Anaesthesia 1990;64:85–104. doi:10.1093/bja/64.1.85
- 23SEMPLE D, RUSSELL S, DOYLE E, et al. Comparison of morphine sulphate and codeine phosphate in children undergoing adenotonsillectomy. Pediatric Anesthesia 1999;9:135–8. doi:10.1046/j.1460-9592.1999.9220317.x
- 24McEwan A, Sigston PE, Andrews KA, et al. A comparison of rectal and intramuscular codeine phosphate in children following neurosurgery. Pediatric Anesthesia 2000;10:189–93. doi:10.1046/j.1460-9592.2000.00482.x
- 25Persson K, Hammarlund-Udenaes M, Mortimer O, et al. The postoperative pharmacokinetics of codeine. Eur J Clin Pharmacol 1992;42:663–6. doi:10.1007/BF00265933
- 26Quiding H, Lundqvist G, Boréus L, et al. Analgesic effect and plasma concentrations of codeine and morphine after two dose levels of codeine following oral surgery. Eur J Clin Pharmacol 1993;44:319–23. doi:10.1007/BF00316466
- 27Twycross G, Wilcock A, Howard P, et al., editors. Drugs and fitness to drive. In: Palliative Care Formulary. London: : Pharmaceutical Press 2020. N/A.
- 28Drug driving: guidance for healthcare professionals. Department for Transport. 2020.www.gov.uk (accessed Apr 2021).
- 29Twycross G, Wilcock A, Howard P, editors. Strong opioids . In: Palliative Care Formulary. London: : Pharmaceutical Press 2020. N/A.
- 30Stolbach A, Hoffman R. Acute opioid intoxication in adults. UpToDate. 2020.https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults (accessed Apr 2021).
- 31Benzodiazepines and opioids: reminder of risk of potentially fatal respiratory depression. Medicines and Healthcare products Regulatory Agency. 2020.https://www.gov.uk/drug-safety-update/benzodiazepines-and-opioids-reminder-of-risk-of-potentially-fatal-respiratory-depression (accessed Apr 2021).
- 32Bandieri E, Romero M, Ripamonti CI, et al. Randomized Trial of Low-Dose Morphine Versus Weak Opioids in Moderate Cancer Pain. JCO 2016;34:436–42. doi:10.1200/jco.2015.61.0733
- 33Sudheer P, Logan S, Terblanche C, et al. Comparison of the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy. Anaesthesia 2007;62:555–60. doi:10.1111/j.1365-2044.2007.05038.x
- 34Management of pain in children. The Royal College of Emergency Medicine. 2017.https://www.rcem.ac.uk/docs/RCEM%20Guidance/RCEM%20Pain%20in%20Children%20-%20Best%20Practice%20Guidance%20(REV%20Jul%202017).pdf (accessed Apr 2021).
- 35Surgery and Opioids: Best Practice Guidelines 2021. Faculty of Pain Medicine, of the Royal College of Anaesthetists. 2021.https://fpm.ac.uk/sites/fpm/files/documents/2021-03/surgery-and-opioids-2021_2.pdf (accessed Apr 2021).

