Wes Mountain/The Pharmaceutical Journal
By the end of this article, you should be able to:
- Understand the correct use of atomic or molecular mass when making dosage related calculations;
- Correctly use stoichiometry to calculate moles and mass;
- Calculate molar mass of compounds in preparations when the molar formula is known;
- Express concentrations in a variety of units.
Introduction
Understanding the basics of stoichiometry is important in solving pharmaceutical calculations involving molar masses and percentage concentrations. When the molecular formula of a compound is known, calculating its molar mass becomes straightforward. The molar mass is the sum of the atomic masses of all atoms in the molecule, expressed in grams per mole (g/mol). This value is essential for converting between moles and grams, which is a common requirement in pharmaceutical preparations.
Concentration can be expressed in various ways, but % w/v (weight/volume) is particularly useful for liquid preparations. It indicates the mass of solute in grams per 100mL of solution. Additionally, expressing concentration as molecular weight per volume helps in understanding the amount of a substance in a given volume, which is crucial for preparing solutions with precise concentrations. To tackle exam questions related to molecular weight, you need to keep track of the aspect of quantity you are working with when you move from units of measure and converting to percentages. It is important to change to common units before working out the final percentages.
The general steps we recommend you follow when working out molecular weight are as follows:
- Write a simple equation to show the dissociation of the compound and the moles involved. For example, for NaHCO3, it would be: NaHCO3 = Na+ + HCO3–;
- Note that the mole ratio is 1: 1; that is 1 mole of NaHCO3 gives 1 mole of Na+ and 1 mole of HCO3–;
- Convert the units of a given drug compound to moles;
- Using the mole ratio, calculate the moles of substance on each side of the equation;
- Convert moles of wanted molecular component to desired units.
These basic steps may appear complicated at first, but with practice, they will all become easier.
Example question
A patient has been prescribed sodium bicarbonate 420mg/5mL; 30mL every 4 hours. The recommended daily allowance for an adult for sodium (Na+) is 2.4g.
Calculate the % of sodium (Na+) above the average daily allowance that is contained in the daily dose of sodium bicarbonate. Give your answer to the nearest whole number.
Atomic weights (g/mole)
Na = 23
H = 1
C = 12
O = 16
Comments and explanations
This question incorporates the concept of stoichiometry and the concentration in terms of weight per volume. You need to be able to calculate how much (in g or mg) of sodium was given to the patient and, to be able to do that, you need to know how many moles of sodium (Na+) would be formed from the dissociation of sodium bicarbonate (1NaHCO3). Any of the molecular components could be selected by the examiner to construct the calculation question. In this question, your focus should be the sodium ion and the sodium bicarbonate in the stoichiometry equation.
Step 1
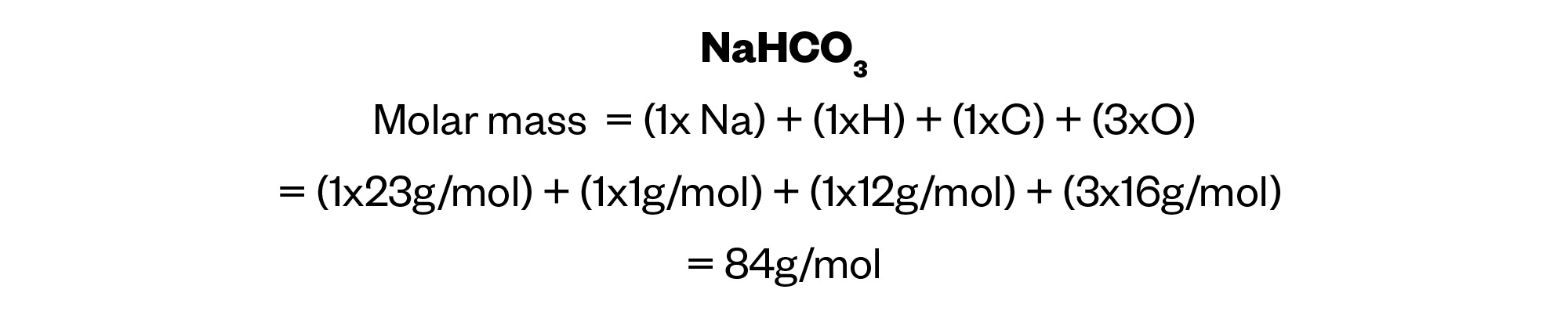
First, we need to calculate the molar mass of sodium bicarbonate, using the molar formula NaHCO3. The atomic weights of the separate elements contained in sodium bicarbonate are listed in the question so it is a simple task to use these weights to calculate the molecular weight of the compound.
Step 2
Calculate the mass of sodium bicarbonate administered to the patient, using the information provided in the prescription.
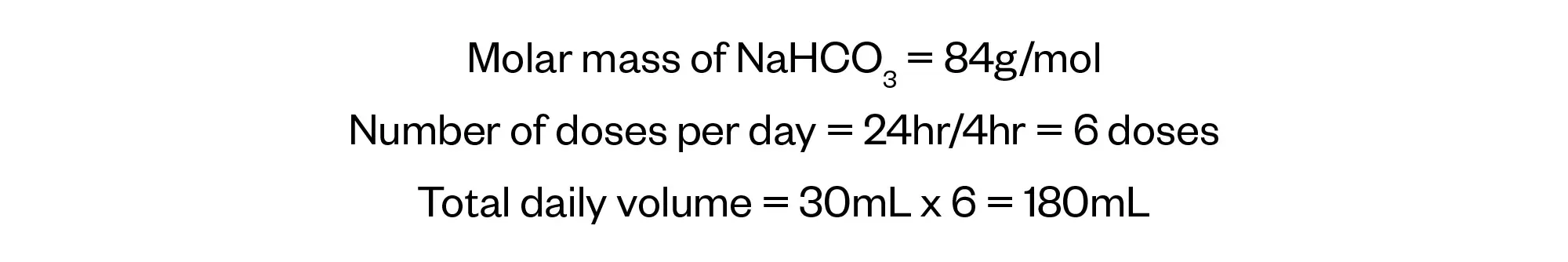
We can now use the concentration of the sodium bicarbonate preparation to relate the volume administered to the molar mass of sodium bicarbonate.
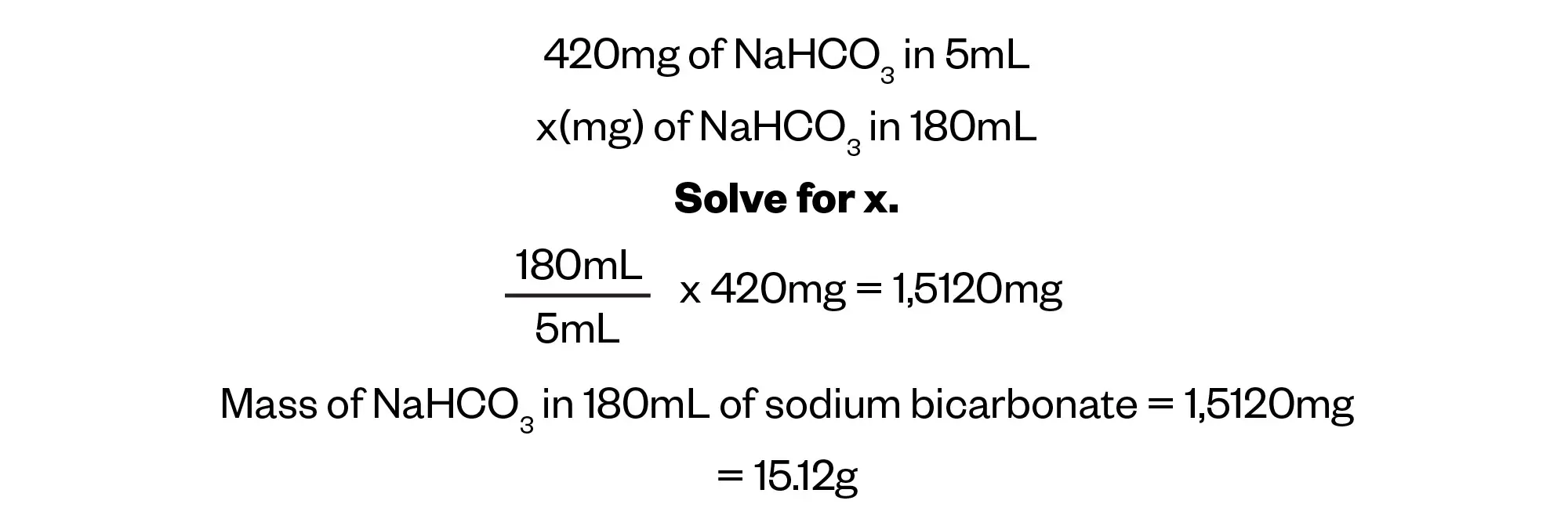
It is important to be fluid in unit conversions, in this case converting mg to g.
Step 3
Calculate the mass of sodium administered to the patient, using the information provided in the prescription.
Knowledge of stoichiometry is important here.
We can now work out the number of moles of NaHCO3 in 180mL of sodium bicarbonate by dividing the daily mass of NaHCO3 prescribed by the molar mass of NaHCO3 that we worked out in Step 1. From there, we can work out the mass of sodium being administered.
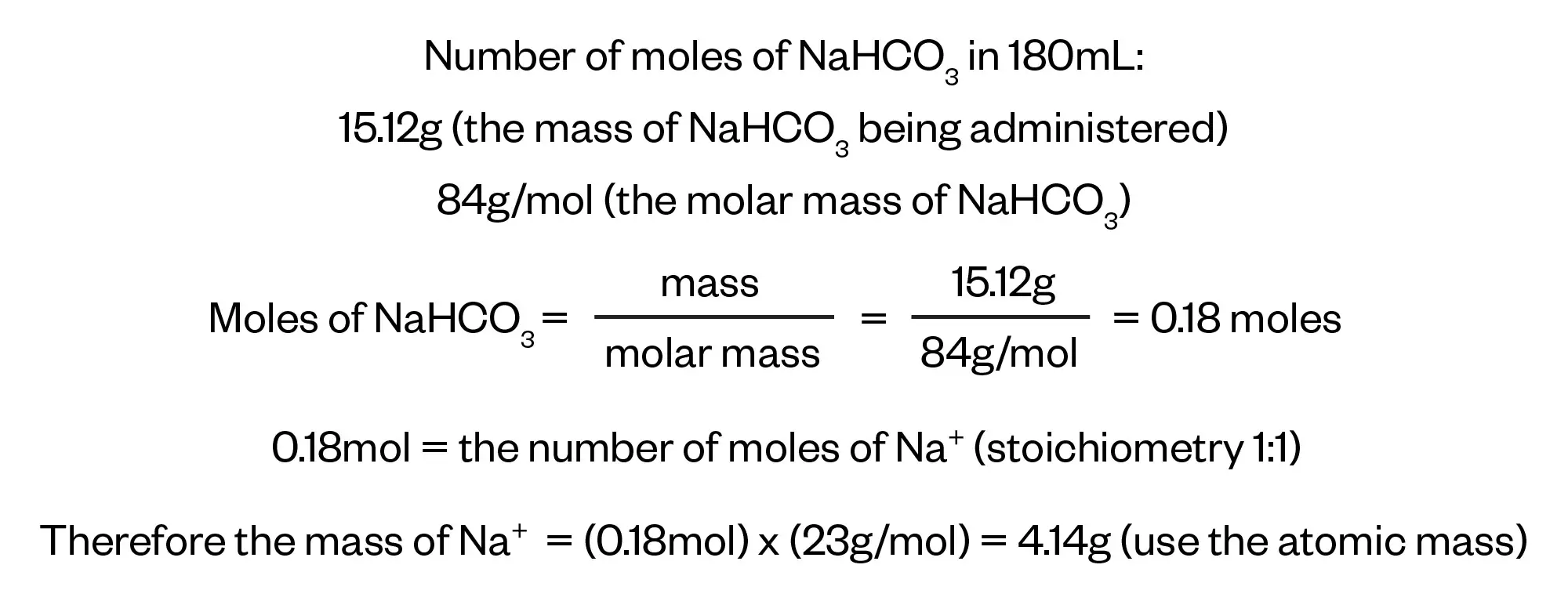
Step 4
Calculate the percentage of sodium prescribed above the recommended daily allowance.
As noted in the question, the recommended daily allowance for an adult for sodium (Na+) is 2.4g and we have now calculated that the patient will be receiving 4.14g from their prescription so the final step is to find the amount of sodium (Na+) above the recommended allowance expressed as a percentage.
That is:
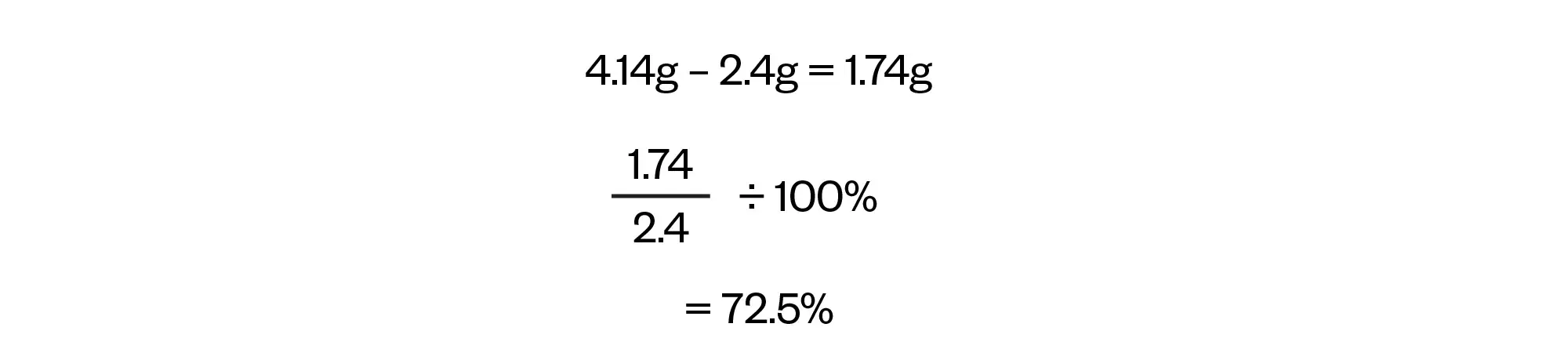
Rounded to 73% to the nearest whole number.
Further reading
The following resources provide more detail on some of the topics introduced in this article:
Acknowledgements
All practice questions were reproduced with kind permission provided by ‘Focus Pre Reg Revision‘.



