Wes Mountain/The Pharmaceutical Journal
By the end of this article, you should be able to:
- Understand how to solve simple and serial dilution calculations;
- Understand unit conversions and the common units used to express concentration;
- Understand the terminology and formulae which are important in solving pharmaceutical dilution scenarios.
Introduction
Dilutions are an important topic for pharmacy calculations. Diluting a medicine changes its concentration allowing the pharmacist to adjust its strength to meet the patient’s specific dosage. When performing dilution calculations, it is important to be competent at unit conversion and be familiar with the various forms of concentrations commonly used when working with solutions.
When answering a dilution question, you should carefully and logically follow the dilution process described in the scenario. It can be helpful to visualise the process by drawing beakers and indicating the volumes and concentrations of each solution. Then, the dilution process can be indicated by arrows from each solution with dilution factors (DF) inserted between the different solutions. We will show how this can be done in the example below.
Failing to understanding dilution terminology is a frequent source of error and therefore it is important to read the question very carefully and understand what is required. For example, an instruction to ‘dilute 10mL to 100mL’ will result in a final volume is 100mL. However, if the instruction was to ‘dilute 10mL with 100mL’ you would end up with a final volume of 110mL. The concept of parts can equally lead to confusion unless well-thought through and understood. The statement, ‘dilute 1 part to 3 parts’ means the final product has 3 parts. This is subtle but important difference to a similar sounding instruction to dilute 1 part of a medicine with 3 parts of water, which will result in 4 parts of final product. Be careful with other dilution scenarios where the wording could be, ‘10mL of drug was dissolved in 140mL of diluent’, meaning the final product is 150mL.
A key tool to use when answering questions on concentrations and dilutions is the formula below which you should know and be comfortable with:
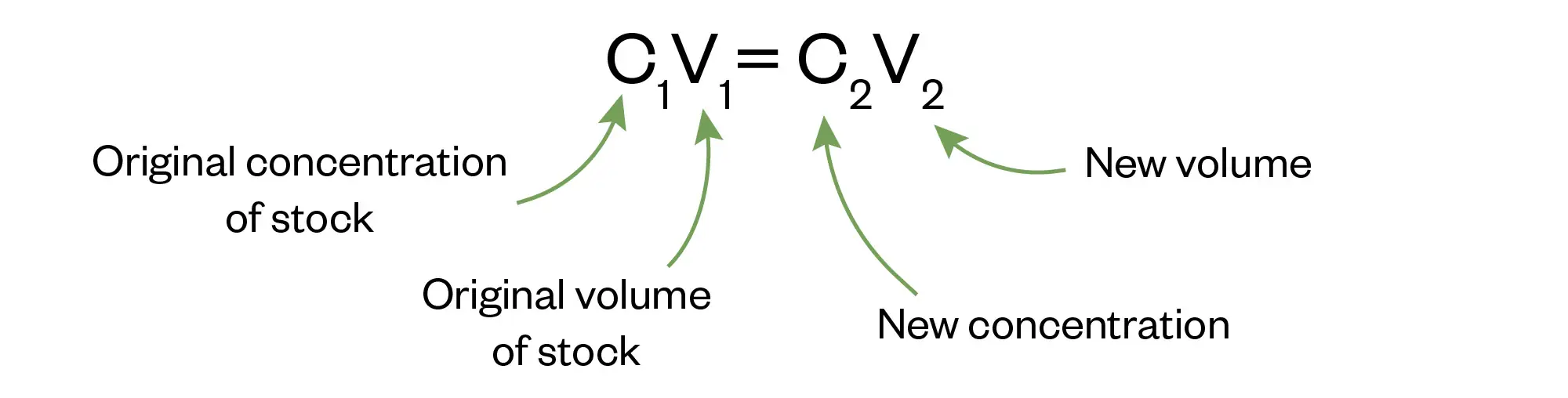
Or its variant, the dilution factor method (DF), where:
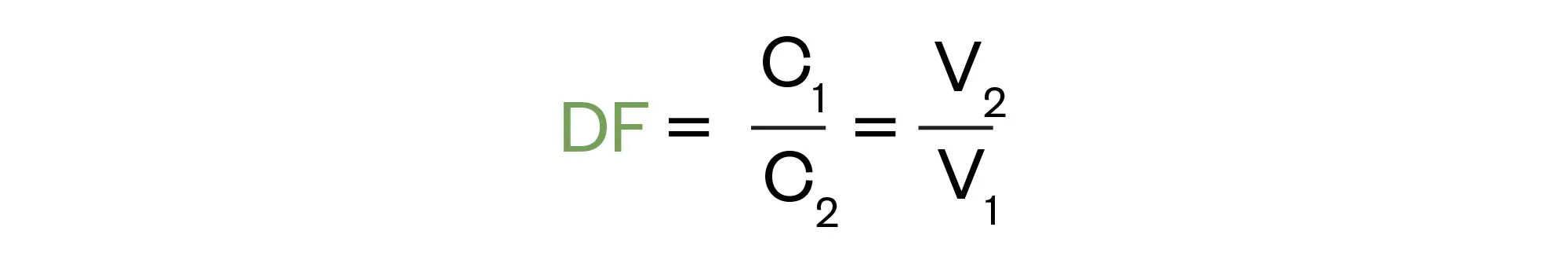
Example question
A stock solution containing a 1 in 5 (w/v) of Drug Y is used to prepare an intermediary solution such that when 2mL of the intermediary solution is diluted to 60mL with water, a 1 in 12,000 solution is obtained.
How much of the stock solution is required to supply 300mL of the intermediary solution? Round your answer to the nearest 0.7mL.
Comments and explanations
This question requires you to apply three distinct knowledge sets in order for you to be able to solve it:
- Being competent in converting concentrations expressed as 1 in X;
- Understanding dilution terminology;
- Applying rounding rules.
You should also have a clear understanding of the dilution process as described by the scenario and here we will use visual representation as an aid.
Step one
Illustrate the dilution being described in the scenario.
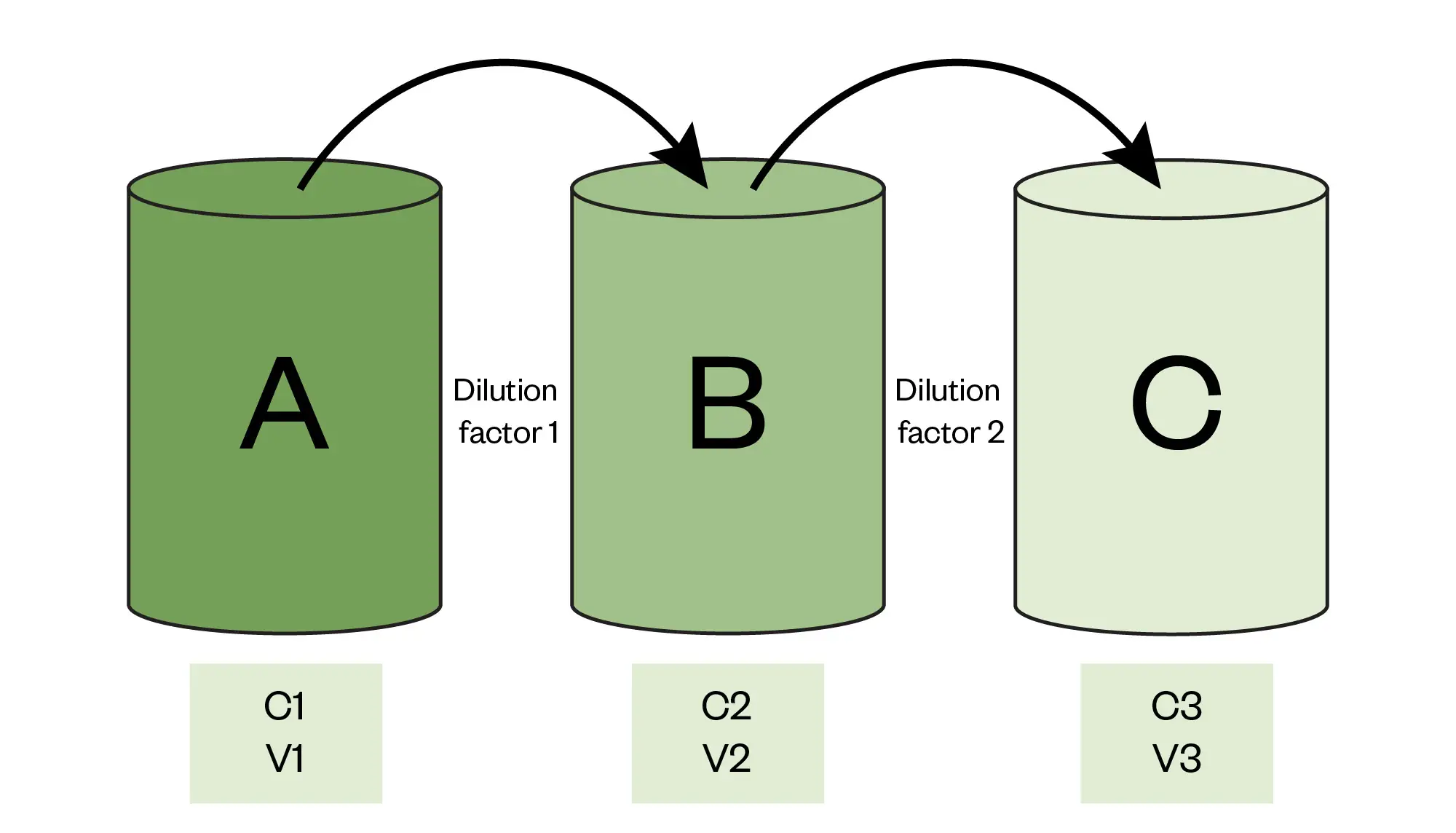
Here we have called the initial stock container A, the intermediate solution container B and the final product container C with arrows showing how the process progresses. There are boxes added for dilution factors, volumes and concentrations which we can begin to fill out using the information contained in the question.
Step two
Write down or calculate the required information and annotate in your illustration.
Now that the question has been visualised, we need to start adding in the respective variables using the details contained in the scenario. Some are provided for us, others we will need to work out.
Before progressing any further, it is important to look at the units being used in the question and perform any necessary conversions. The volumes are all expressed in millilitres (mL) so they do not require any conversion. However, the concentrations have been expressed in two forms: 1 in 5 (w/v) for C1 and 1 in 12,000 for C3. Our approach here is to convert these to percentages (w/v). This gives us the following:
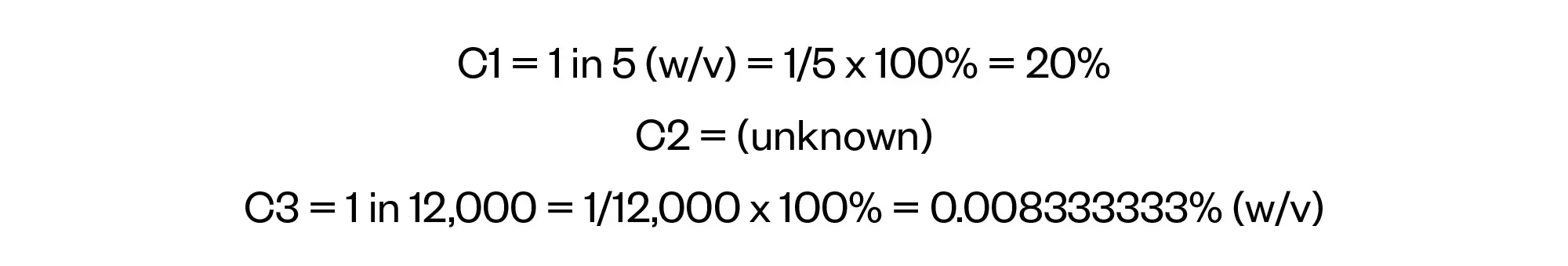
We can also start to add in details for the volumes.

From the information in the question, we are also able to identify the second dilution factor (from B to C) as we know that 2mL of B is diluted to 60mL to give C. Using the dilution factor equation provided above this can be calculated as:
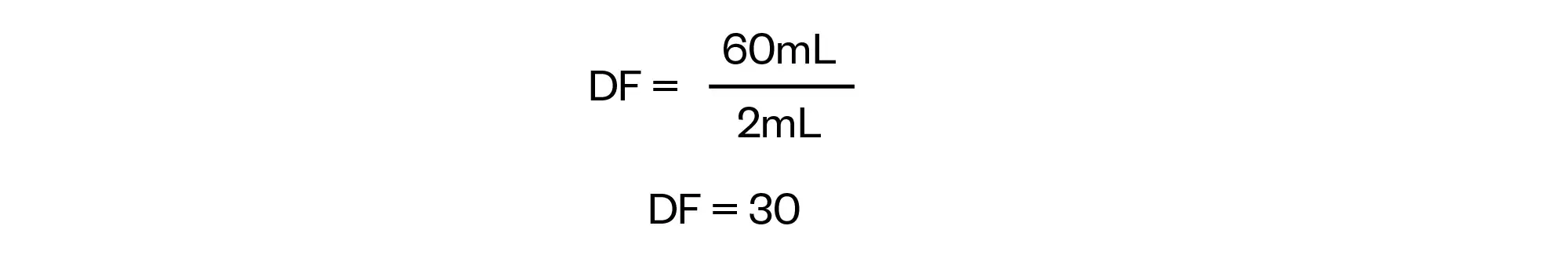
Adding these details to our visual representation gives us the following:
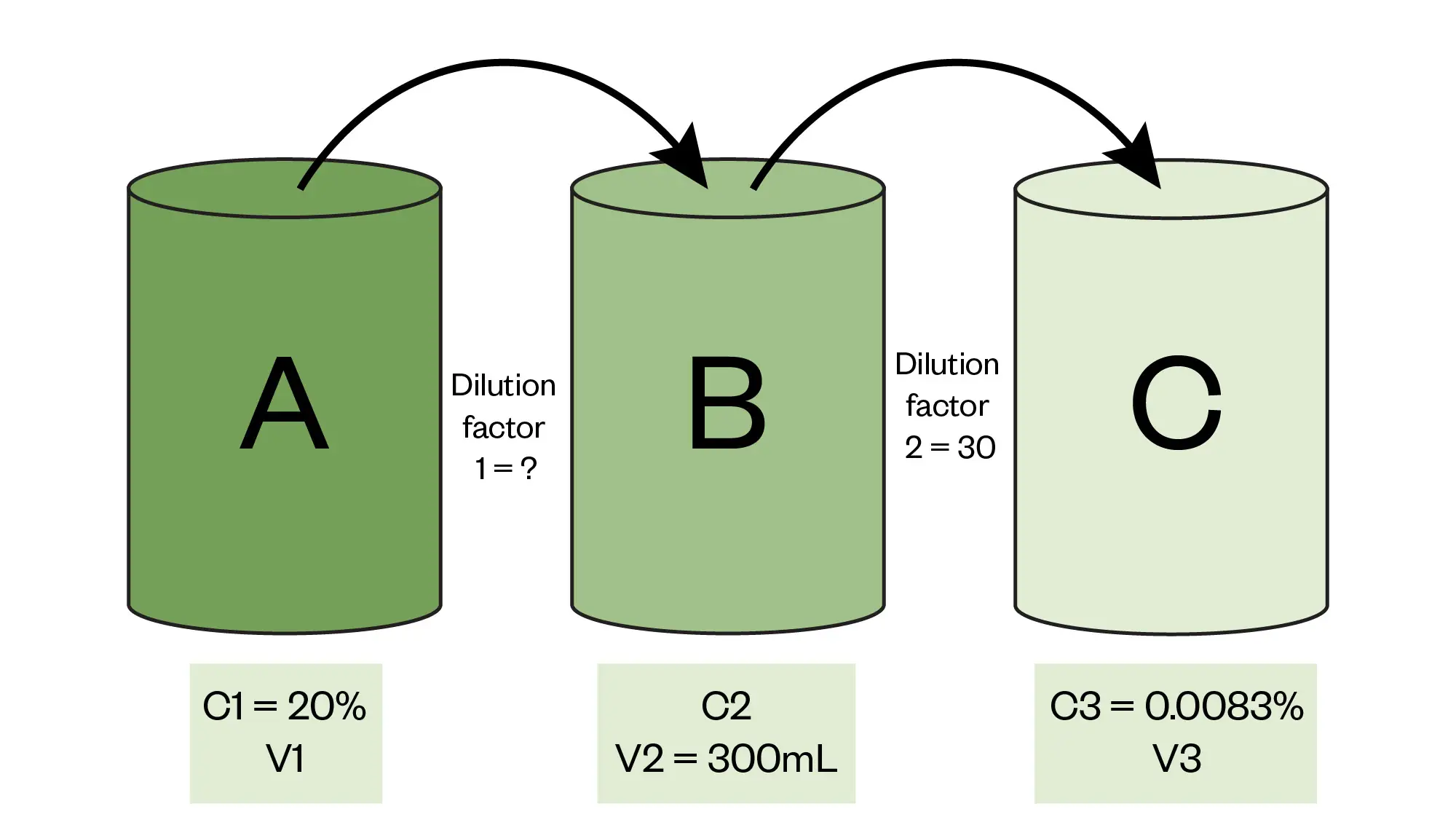
Step three: calculate missing information for C2
Now that we have the second dilution factor it is a simple task to calculate the concentration of intermediary B, again by rearranging the formula provided above.
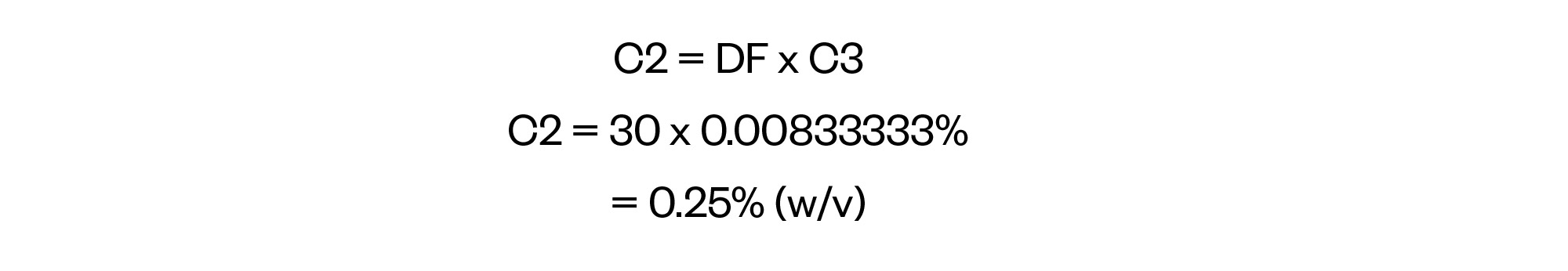
Step 4: Calculate V1
We now have sufficient information to be able to perform the final calculation necessary to answer the question by using C1V1= C2V2.
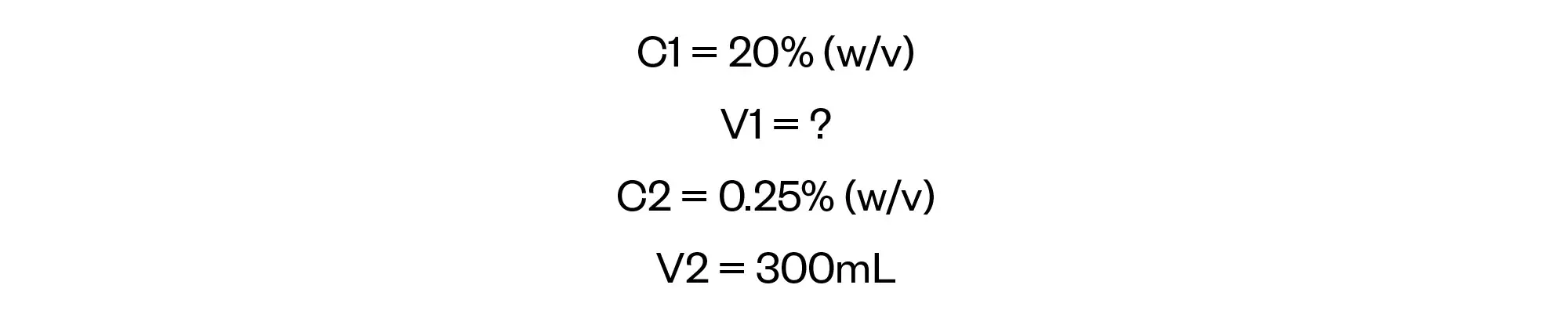
Rearrange C1V1= C2V2 to solve for V1:
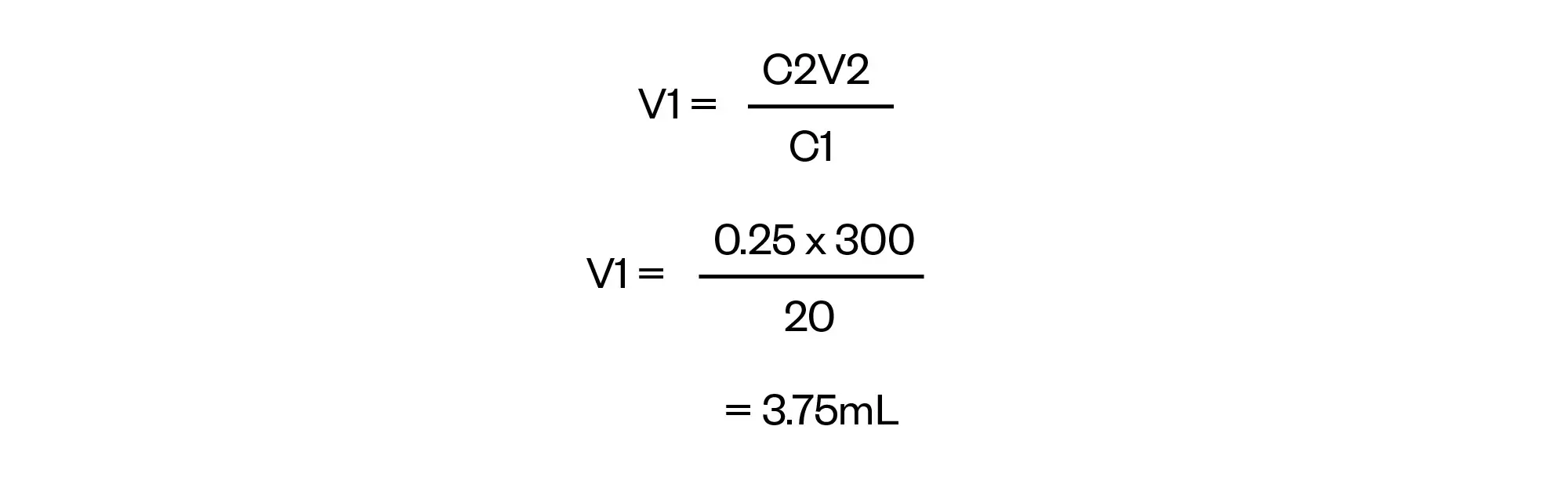
Finally, note that the question asked for the answer to be rounded to the nearest 0.7mL.
Final answer = 3.5mL
You could have alternatively focused on the first dilution factor (from A to B) as a strategy for this final calculation. We could also have continued to work out further details such as V3 using the formulas in a similar way but it is important to be efficient with your use of time during the exam and focus on steps that align with what the question asks for. In this example, working out V3 would have been irrelevant and wasted valuable time.
Disclaimer
The views in this article are those of the authors and do not represent the views of any organisations they are associated with. The questions and explanations presented here are for educational purposes only and do not replace your training, knowledge and application of professional judgement as a pharmacist or trainee pharmacist. The example questions used in this article and the answers provided are for educational purposes and should not be translated to represent what would happen in real practice.
Acknowledgements
All practice questions were reproduced with kind permission provided by ‘Focus Pre Reg Revision’