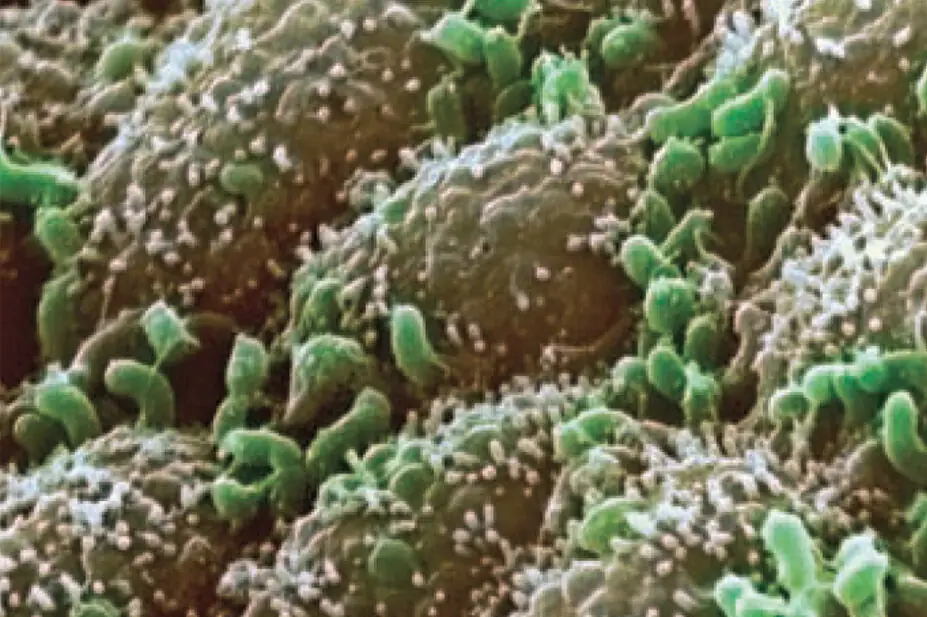
BIOMEDICAL IMAGING UNIT, SOUTHAMPTON GENERAL HOSPITAL/SCIENCE PHOTO LIBRARY
Humans and animals are home to a diverse array of bacteria whose numbers dwarf even the number of cells in the body. They are referred to as the microbiome and their various roles and functions in health and disease represent a significant avenue of investigation.
Readers may have noticed an advertisement appearing in recent issues of The Journal. This advertisement is not for a new medicine or a new formulation but rather for something that people can pick up from the dairy section of their local supermarket: a probiotic yoghurt drink.
Yoghurt drinks containing probiotics (micro-organisms thought to confer a health benefit to the host) carry some reference to science, in particular how the specific bacterial content of the drink may help reduce the incidence of antibiotic-associated diarrhoea and diarrhoea attributed to Clostridium difficile infection in specific populations.1 such research represents part of a wave of increasing interest in how the trillions of bacteria that call the human gut home may have the propensity to do so much more than help digest our food.
On 13 June 2012 first results from the Human microbiome Project were published.2 The project aimed to map the multitude of bacteria that inhabit us. Looking at various areas of the body ranging from the nasal and oral cavities to the gastrointestinal and genitourinary tracts, researchers published volumes of data on the different types of bacterial species present. They also speculated on their role in health and illness. The science of microbiomics —the study of the collected bacteria that populate an organism — had its red-letter day.
In this article we are going to focus on one part of the microbiome, the gut microbiota, estimated to carry over 70 per cent of our microbial passengers, and how these bacteria may well hold some interesting clues to health and disease. To coin a phrase from Hippocrates (400BC) ,“death sits in the bowels”. This demonstrates some of the earliest views on the importance of the human GI tract beyond just digesting food. Nowadays, the bacterial contents of the human GI tract are fast becoming a source of some significant scientific inquiry.
Echoing the sentiments of Lupp and colleagues3 that “now is an exciting time to study the microbes that are associated with the body — the human microbiota”, both medical science and technological advancement are converging toward some potential key roles of bacteria in health. Based on various themes, such as the interaction between the gut microbiota and immune function, the involvement of GI bacteria in disease and the possibility that gut bacteria might be able to influence behaviour, science is starting to take gut bacteria seriously.
Gut bacteria and immune function
Considering the diverse array of bacteria that make up the human gut microflora and that spread across various sites of the GI tract, due credit should be given to the way that the body manages, and is normally able, to tolerate such residents over the course of a lifetime. The GI tract comprises various aerobic, anaerobic and facultative species. A dynamic relationship exists between the various phyla and species of bacteria resident in the GI tract, which is affected by our genetics, our earliest days of microbial exposure (eg, the birth canal) and modifiable factors such as diet, environment and Gut bacteria are now known to do more than just digest food. Maynard and colleagues4 summarised the reciprocal relationship between our gut microbiota and immune system, where both systems engage in continual dialogue. Beyond mucosal immunity, current opinion suggests that the gut microflora may also show involvement with more systemic immunity based on gnotobiotic (germ-free) animal studies,5 for example. Cucchiara and colleagues6 discussed how, using the example of paediatric inflammatory bowel disease (IBD), the interaction between the gut microbiome and the innate immune system can sometimes turn destructive. They, alongside other authors,7 discussed the potential modifying capability of gut bacteria with regard to symptoms such as inflammation. Such research was also complemented by the suggestion that early use of antianaerobic antimicrobials may also modify later risk of IBD.8
Gut microflora and disease
When bacteria and the human host are in harmony, benefits are present for both. Bacteria get a nutrient-rich home to thrive and multiply while their human hosts benefit from their digesting and nutrient-supplying capabilities. When, however, problems arise in that relationship, the effects can be farreaching, particularly as a consequence of a state of dysbiosis — a disruption to the normal balance of various bacteria.
Duboc and colleagues9 concluded that gut dysbiosis interferes with normal bile acid metabolism, which itself impacted on the anti-inflammatory functioning and the cycle of inflammation noted in IBD. Other GI-based conditions have similarly been linked to abnormal gut microflora profiles, including coeliac disease,10 which leads to the relationship between gut bacteria and autoimmunity11 and possibly even gut barrier function.12 Various other conditions are also being linked to issues with gut microflora, including obesity and diabetes. Perhaps even more interesting are the suggestions that modifying the gut microbiota with antibiotics and probiotics may impact on the presentation of such conditions. Another potential therapeutic option is also currently finding some favour: faecal transplantation or faecal bacteriotherapy (taking a stool from a healthy donor, blending and repackaging it for delivery into another person via enteric-coated capsule or nasogastric tube). Disgust aside, important results for the treatment of C difficile infection are being reported from faecal transplants.13
More than just butterflies in the stomach?
Of perhaps even greater significance is the suggestion of a role for the gut microflora beyond the digestive tract to affecting overt behaviour. Cryan and Dinan14 discussed this possibility of a “microbiota-gut-brain axis” where gut microflora may potentially influence pain, anxiety and even cognition.
There is emerging evidence for a bidirectional role for gut bacteria and behaviour in various animal models,15,16 although there is rather less evidence in humans. That being said, the gut microflora are receiving considerable research attention in a variety of behaviourally led conditions, particularly cases of autistic spectrum disorders.17,18
Gut bacteria affecting medication
Oral dosage forms continue to enjoy a privileged position in medicines delivery. Depending on the formulation, drug absorption at various sites of the GI tract will inevitably bring medicines into contact with the gut microbiota. The question is whether the gut microflora can affect drug delivery and effectiveness.
Yes, said Mikov,19 who suggested that “gut flora metabolism must be considered an integral part of drug metabolism and toxicity studies”. Indeed, there are multiple examples of gut bacteria and its metabolites potentially affecting the efficacy of oral pharmacotherapy.
Kaddurah-Daouk and colleagues20 reported on a correlation between the presence of several bile metabolites produced by specific strains of GI bacteria and the effectiveness of simvastatin in lowering low-density lipoprotein cholesterol.
Clayton and colleagues,21 in a similar vein, observed that another bacterial by-product, para-cresol, might influence paracetamol metabolism. This and other work generally collected under the label of pharmacometabonomics22 show how an individual’s gut microflora should be regarded as an important part of the world of medicines testing and, indeed, individual responses to medicines.
Conclusions
Given the size and complexity of the bacterial population housed in the human gut, one would perhaps expect their effects to be more wide-ranging than merely aiding the digestion of food. The emerging concepts of gut microflora interacting with the immune system and other seemingly distant organs such as the brain offer some unique opportunities to science and medicine.
Kalliopi Dodou is senior lecturer in pharmaceutics, Sunderland Pharmacy School, University of Sunderland.
Paul Whiteley is a researcher based in the north east of England. Correspondence to: Kalliopi Dodou (email kalliopi.dodou@sunderland.ac.uk)
The original PDF of this article can be found here.
You might also be interested in…

UK Clinical Pharmacy Association launches pharmacogenomics guidance
Health news round-up: cardiovascular disease, women’s health and the potential for new sepsis treatment
