Summary
Management of myasthenia gravis (MG) can be divided into four main areas: avoidance and management of disease triggers; symptomatic treatment; immunosuppressant drugs; and immunomodulatory treatments, such as use of intravenous immunoglobulin or plasma exchange.
Anticholinesterase medicines are used for symptomatic treatment of MG. They increase the amount of acetylcholine at the neuromuscular junction, thereby improving muscle strength. Corticosteroids are the most commonly used immunosuppressant for MG, but some patients will also require medicines such as azathioprine, methotrexate, mycophenolate or tacrolimus.
Management of myasthenia gravis (MG) can by divided, broadly, into four main categories: avoidance and management of disease triggers; symptomatic treatment; immunosuppressive therapy; and immunomodulatory therapy.
Avoiding triggers
The following factors can exacerbate muscle weakness in patients with MG or trigger myasthenic crisis (and should therefore be avoided if possible):
- Infection
- Stress or trauma
- Surgery
- Thyroid dysfunction
- Withdrawal of cholinesterase inhibitors (if symptoms are not fully controlled)
- Rapid introduction or increase of corticosteroids
- Anaemia
- Electrolyte imbalance (for example, hypokalaemia or hypermagnesaemia)
- Medicines (see below)
Medicines
Several medicines have the potential to worsen the symptoms of MG and these should be avoided unless there is no alternative (see Box 2, p289). If these medicines are required, they should be started and titrated cautiously. There are also some medicines that are contraindicated in MG, eg, botulinum toxin. Medicines that can worsen MG symptoms are generally those that:
- Interfere with neuromuscular transmission, thereby unmasking or worsening symptoms
- Increase muscle weakness more generally, including those that do so by causing electrolyte disturbances
Medicines, particularly penicillamine, have also been associated with development of MG, supposedly by initiating an inappropriate immune response. In documented cases the onset of symptoms occurred between two and 12 months after starting penicillamine and, in most cases, was followed by complete resolution within six months of stopping the drug.5 Methyldopa and interferon alfa have also been associated with the onset of MG.
At the time of diagnosis, and during exacerbations, a detailed medication history should be obtained so that any possible trigger drugs can be identified and, if possible, stopped. Patients should also be advised to remind anyone involved in their care that they have MG and to ask whether or not new medicines could affect their condition.
Symptomatic treatment
In MG defective acetylcholine (ACh) signalling in neuromuscular junctions results in muscle weakness. In normal tissue, an accumulation of ACh in the synaptic cleft is prevented by the presence of the enzyme acetylcholinesterase, which breaks down the ACh. For patients with MG, inhibiting acetylcholinesterase (using anticholinesterases) can boost the concentration of ACh in the synapse and overcome deficits in postsynaptic signalling.
Although there are no randomised controlled trials of anticholinesterases for MG, their use is well established. A Cochrane review concluded that the response to anticholinesterases is so clear that it would be difficult to justify a randomised controlled trial that deprived participants allocated to a placebo arm.6
Several anticholinesterases are available for the treatment of MG, namely distigmine, edrophonium, neostigmine and pyridostigmine. In practice only edrophonium and pyridostigmine are used, for diagnosis (see accompanying article, p282) and treatment, respectively.
Pyridostigmine is preferred for treatment because it has a longer duration of action than neostigmine and less potential to cause cholinergic crisis than distigmine.
The adverse effects of anticholinesterases are largely dose-dependent and predictable, and can divided into nicotinic and muscarinic effects. Nicotinic side effects include muscle and abdominal cramps. Skeletal muscles can also twitch and, if this happens at a high enough rate, short-term paralysis can occur in the affected muscle (although this is generally only seen in overdose).
Common muscarinic side effects include gut hypermotility (with abdominal cramps or diarrhoea) and increased sweating, salivation or lacrimation.
Uncommon muscarinic side effects include hypotension, bradycardia, miosis, urinary incontinence, increased bronchial secretions and tachypnoea. Generally these effects are dose-dependent and are therefore more likely in patients receiving high doses of anticholinesterases, or following overdose.

Box 1: Immunomodulatory therapies
Immunomodulatory therapies are indicated for the management of myasthenic crisis, or in patients with severe myasthenia gravis (MG) that is poorly controlled with other therapies.
Intravenous immunoglobulin (IVIg) suppresses the autoimmune processes in MG. Although its precise mechanism of action is unclear, it probably involves increased removal of harmful antibodies and modification of the immune response. Typically, the dose of IVIg is 0.4g/kg daily for five days. Plasma exchange, in which circulating antibodies are removed through membrane filtration or centrifuge, is also a treatment option for patients with MG.
According to Cochrane reviews, the use of IVIg or plasma exchange can provide short-term benefit for patients with MG, particularly those with myasthenic crisis.1,2 Generally, improvements occur in the first week of therapy and last between one and three months. The limited evidence does not support repeated administration to obtain or maintain lasting response, but some patients who do not respond to oral therapies may require IVIg or plasma exchange frequently.
Both options are associated with similar outcomes although IVIg may be better tolerated and also has the benefit that it can be used in the presence of infection — which is important since sepsis can often be a trigger for myasthenic crisis. Because IVIg is a human-derived blood product there may be issues with its use in certain patient groups such as Jehovah’s Witnesses. The use of IVIg is also managed as part of the Department of Health national demand management programme for immunoglobulin. Within the confines of this programme clinicians may use IVIg to manage disease flares only when they require hospital admission.3
To reduce the risk of adverse effects pyridostigmine should generally be introduced slowly, and an optimal dose determined by balancing clinical improvement and side effects — both of which can vary over time and depending on concomitant treatments. In the UK, pyridostigmine is available as a scored 60mg tablet, which can be quartered. A typical regimen would start at 15mg four times a day, increasing after two days to 30mg four times a day, and then after a further two days to 60mg four times a day (a typical maintenance dose is 60mg four to six times a day).
Patients with MuSK antibodies (see accompanying article, p282) often have ACh receptor hypersensitivity, so smaller doses and slower titration are advised. Although these patients are more sensitive to the effects of anticholinesterases, the maximum benefit they experience is often less than for other serotypes.
The maximum licensed dose of pyridostigmine is 1.2g daily, but the British National Formulary advises against daily doses above 450mg because of the risk of receptor down-regulation. In practice, single doses above 60mg and daily doses above 360mg are rarely used. If symptoms remain poorly controlled at these doses, adding immunosuppressant therapy should be considered.
If patients experience problematic muscarinic side effects an antimuscarinic medicine can be prescribed (eg, propantheline 15mg, taken 15–30 minutes before each pyridostigmine dose).

Immunosuppressant therapy
Immunosuppressive treatments target the immune response; the aim is to induce and maintain remission of MG. Observational studies show that such treatments bring about remission or marked improvement in 70–80% of patients.7
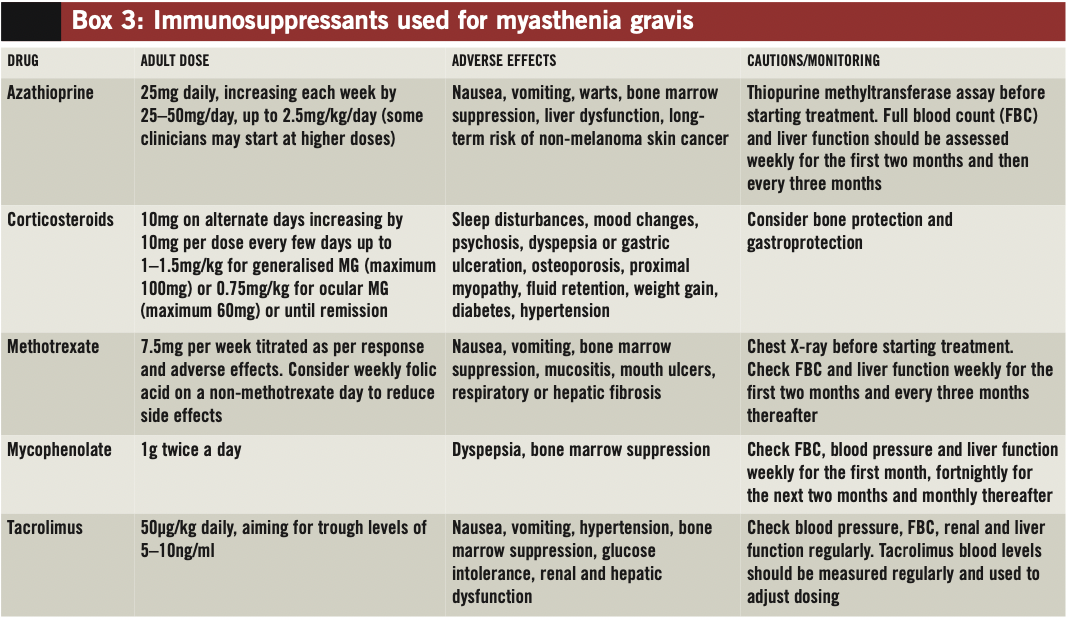
Corticosteroids, generally prednisolone, are the mainstay of immunosuppressant therapy for MG. Other medicines, such as azathioprine, ciclosporin, methotrexate, mycophenolate or tacrolimus, are generally considered second-line options and can be used for their corticosteroid-sparing effects (see below and Box 3, p290). Evidence supporting the use of these immunosuppressants for MG is limited and, in some cases, inconclusive.8 Although there are data for ciclosporin and cyclophosphamide, these medicines are rarely used because of concerns around toxicity (namely, renal impairment and hypertension with ciclosporin and bone marrow suppression, bladder toxicity and malignancy with cyclophosphamide).
Generally, once a patient has achieved remission with an immunosuppressant (which typically occurs after four to 16 weeks) symptomatic treatment, ie, pyridostigmine, should be withdrawn slowly over two to four weeks, with careful observation for recurrence of symptoms. (Reintroduction of symptomatic treatment can be required during disease flares.)
Corticosteroids
There is evidence that corticosteroids provide symptomatic improvement for patients with MG;5 generally, oral prednisolone is the corticosteroid of choice in the UK.9
The adverse effects of corticosteroids are well established and can be marked. For patients with MG, starting high-dose corticosteroids can cause a transient worsening of symptoms (typically after four to 10 days) and can precipitate myasthenic crisis.9
There is no consensus around how best to start and titrate prednisolone. Most neurologists start at 10mg every other day, increasing by 10mg every few days until symptoms resolve or the target dose is reached. The typical target dose is 1–1.5mg/kg, up to a maximum of 100mg, every other day (for patients with ocular symptoms only, the target dose tends to be lower — 0.75mg/kg, up to a maximum of 75mg, every other day). This alternate-day regimen is widely used because it is believed to limit severe side effects.10 An important exception to this is in patients with diabetes, for whom daily dosing is preferred because alternate-day dosing can cause fluctuations in blood sugar and compromise diabetic control.
At the beginning of treatment with the alternate-day regimen, patients occasionally feel worse on the days that they do not take corticosteroids. If this early fluctuation occurs, patients can be switched to a daily regimen;11 however, early fluctuation seems to be a good indicator of corticosteroid response and usually settles with time.10
There is also debate around starting treatment with high daily doses of corticosteroid in critically ill patients. The approach is recommended in guidance issued by the European Federation of Neurological Societies, in which additional short-term or supportive therapies to overcome temporary worsening are recommended.7
Another view is that, since corticosteroids typically take weeks or months to work and there is no evidence to start treatment aggressively in severe cases, they should be introduced gradually for all patients; if this approach is taken, intravenous immunoglobulin or plasma exchange should be used to establish an early response (see Box 1, p288).10
Patients starting high-dose corticosteroids should be offered a proton pump inhibitor for gastroprotection. Appropriate bone protection should also be considered for these patients.
Azathioprine
Azathioprine inhibits synthesis of DNA and RNA and interferes with T cell function. It is the most commonly used corticosteroid-sparing medicine for MG.
The onset of action of azathioprine is slow — it takes around four to 12 months before a therapeutic response is seen, and up to 24 months for maximal effect. The drug is often started at the same time as prednisolone to allow the corticosteroid to be tapered over time to the lowest possible dose.
Azathioprine is typically started at a dose of 25mg daily, and titrated up to 2.5mg/kg/day (either as a single or divided dose) — although some clinicians may initiate at higher doses. Treatment is generally well tolerated, although idiosyncratic flu-like symptoms and gastric disturbances occur in around 10% of patients, generally within the first few days of treatment.
Rarely azathioprine is associated with serious adverse drug reactions, such as pancreatitis, bone marrow suppression, liver dysfunction or hepatitis. Close monitoring for such reactions is recommended.
Concurrent use of xanthine oxidase inhibitors (allopurinol or febuxostat) should be avoided because these medicines inhibit azathioprine metabolism. If they are required, the dose of azathioprine should be reduced by 75%.
Another consideration before starting azathioprine treatment is genetic variation in the enzyme thiopurine methyltransferase (TMPT), which is responsible for metabolism of the drug. Reduced activity of this enzyme affects around 10% of the population, and patients should be tested for this before starting treatment.
Although the risk of malignancy with azathioprine is controversial, it is known that immunosuppressed patients have an increased risk of skin cancer.12 Prescribers should discuss risks and benefits of treatment with patients. The manufacturers recommend that patients limit their exposure to ultraviolet light, wear protective clothing and use a sunscreen with a high protection factor to minimise the risk of developing skin cancer.
Methotrexate
Current EFNS guidelines recommend that methotrexate should be considered for patients with MG who do not respond to azathioprine.7 In a recent study comparing daily oral azathioprine with weekly oral methotrexate, both drugs demonstrated a similar corticosteroid-sparing effect within 10–12 months, with similar efficacy and tolerability.13
Folic acid (5mg weekly) is often co-prescribed with methotrexate to reduce the risk of side effects. This should be taken on a different day of the week to that of the methotrexate.
Mycophenolate
Mycophenolate inhibits purine synthesis and has effects on both T cells and B cells. It has been used for the treatment of a range of immune- mediated conditions, but evidence for its effectiveness in MG is conflicting.7,8
Despite these limitations mycophenolate is occasionally tried for patients who do not respond to other immunosuppressants.
Tacrolimus
Tacrolimus has a similar mode of action to ciclosporin, inhibiting proliferation and activation of T cells. There is a small number of case reports suggesting that MG patients could benefit from tacrolimus, and that it is reasonably well tolerated.14
Evidence suggests that the drug could be particularly useful for patients with a specific subclass of antibodies (ie, antibodies to ryanodine receptors, which are involved in calcium release from sarcoplasmic reticulum and potentiate excitation-contraction coupling in skeletal muscle). Current guidelines recommend that tacrolimus be considered for patients with poorly controlled MG, particularly those with ryanodine antibodies.7
References
- Gajdos P, Chevret S, Toyka KV. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database of Systematic Reviews 2008; issue 1.
- Gajdos P, Chevret S, Toyka KV. Plasma exchange for generalised myasthenia gravis. Cochrane Database of Systematic Reviews 2002; issue 4.
- NHS. National demand management programme for immunoglobulin. www.ivig.nhs.uk/clinicinfo.html (accessed 2 July 2012).
- Keogh MJ, Findlay JM, Leach S, et al. Statin-associated weakness in myasthenia gravis: a case report. Journal of Medical Case Reports 2010;4:61.
- Andonopouls AP, Terzis E, Tsibri E, et al. D-penicillamine induced myasthenia gravis in rheumatoid arthritis: an unpredictable common occurence? Clinical Rheumatology 1994;13:586–8
- Mehndiratta MM, Pandey S, Kuntzer T. Acetylcholinesterase inhibitor treatment for myasthenia gravis. Cochrane Database of Systematic Reviews 2011; issue 2
- Skeie GO, Apostolski S, Evoli A, et al. EFNS Guidelines for treatment of autoimmune neuromuscular transmission disorders. European Journal of Neurology 2010;17:893–902.
- Hart IK, Sathasivam S, Sharshar T. Immunosuppressive agents for myasthenia gravis. Cochrane Database of Systematic Reviews 2007; issue 4.
- Schneider-Gold C, Gajdos P, Toyka KV, et al. Corticosteroids for myasthenia gravis. Cochrane Database of Systematic Reviews 2005; issue 2.
- Hilton-Jones D, Palace J. The management of myasthenia gravis. Practical Neurology 2005;5:18–27.
- Jacob S, Viegas S, Lashley D, et al. Myasthenia gravis and other neuromuscular junction disorders. Practical Neurology 2009;9:364–71.
- Martindale: The Complete Drug Reference. London: Pharmaceutical Press; 2011.
- Heckmann JM, Rawoot A, Bateman K, et al. A single-blind trial of methotrexate versus azathioprine as steroid-sparing agents in generalised myasthenia gravis. BMC Neurology 2011;11:97.
- Tada M, Shimohata T, Tada M, et al. Long-term therapeutic efficacy and safety of low-dose tacrolimus (FK506) for myasthenia gravis. Journal of the Neurological Sciences 2006;247:17–20.
You might also be interested in…
Palliative care: end-of-life medicines management

Palliative care: principles and pharmacy roles