Luca DiCecco / Alamy
Summary
There are no pharmacological treatments that are known to prevent or cure osteoarthritis. Current management is directed at providing symptomatic relief. Pharmacological treatments are adjuncts that offer at best moderate symptom relief and the key to management is a combination of this with lifestyle change. Exercise regimens should include local muscle strengthening (e.g. quadriceps muscle exercises for knee OA) combined with general aerobic fitness. Ideally, patients should have an individualised exercise programme they can perform daily.
International guidelines recommend the use of topical non-steroidal anti-inflammatory drugs (NSAIDs) and/or paracetamol as the first-line treatment of choice. If these are inadequate, the patient should stop their current therapy and use an oral NSAID (either a non-selective or cyclo-oxygenase 2 inhibitor). Patients using oral NSAIDs should also be prescribed a proton pump inhibitor.
The use of opioids is supported in current guidance from the National Institute for Health and Care Excellence, but no reference is made to individual drugs. Opioid analgesics may be indicated for some patients with unacceptable pain despite treatment with oral paracetamol or topical NSAIDs when oral NSAIDs or COX 2 inhibitors are contraindicated.
Osteoarthritis (OA) has a broad range of presentations and therefore its potential impact on a patient’s personal life and employment can vary significantly. The management of OA must therefore be personalised and may include both pharmacological and non-pharmacological interventions. This approach has been shown to provide patients with better pain and functional outcomes[1]
.
The pain and disability associated with OA can have a significant impact on mood and may impair participation in both work and recreational activities. Patients should be educated about their OA and be provided with a tailored self-management plan to maintain physical function and fitness, reduce pain and prevent further deterioration.
Non-pharmacological management
Behavioural changes, such as weight loss, regular exercise, appropriate footwear and the use of aids, can be of benefit to patients with OA.
Exercise can reduce pain and improve overall function in people with hip or knee OA. The National Institute of Health and Care Excellence (NICE) recommends exercise as a core treatment of OA irrespective of age, comorbidity, pain severity or disability.
Exercise regimens should include local muscle strengthening (e.g. quadriceps muscle exercises for knee OA) combined with general aerobic fitness. Ideally, patients should have an individualised exercise programme they can perform daily. The regimen needs to be continued long term and become part of the person’s usual lifestyle rather than an additional task they are required to perform. Some patients with knee OA pain may initially be unable to perform exercises such as a straight leg raise; in these circumstances, it may be better to start with low impact exercises, such as an exercise bike or walking laps in a swimming pool, before progressing to higher intensity exercise.
The European League Against Rheumatism (EULAR) recommends patients with OA should perform moderate-intensity aerobic training for at least 30 minutes per day (or up to 60 minutes for greater benefit), and progressive strength training involving the major muscle groups at least two days per week at moderate to vigorous intensity for ten repetitions[2]
.
Weight loss is important for overweight patients with OA, as obesity is a known risk factor for the development of both hip and knee OA[3]
. Studies in patients with knee OA have shown that supervised weight loss programmes demonstrate small but significant improvements in pain and physical function[1],[4]
. Overweight patients with OA should be educated on weight loss and provided with support to achieve and maintain their target weight. A healthy diet should be encouraged, with limited fat and salt intake and at least five portions of fruit and vegetables per day.
Physical aids can be used by patients with OA. Patients with hip or knee OA should be advised on the use of appropriate footwear to reduce pain and improve physical function; EULAR defines ‘appropriate’ as no raised heel, thick, shock absorbing soles, support for the arches, and a shoe size big enough to give comfortable space for the toes[1]
. Specialised footwear is not required.
There is a lack of evidence to support the use of specific shoes or insoles in patients with hip OA. However, studies involving patients with knee OA have demonstrated shock absorbing insoles worn for one month reduced pain and improved physical function[5]
. Athletic shoes with custom-made insoles and conventional athletic shoes have both been found to reduce pain in patients with knee OA[6]
.
A single randomised controlled trial concluded that using a walking stick, in comparison with usual disease management, can diminish pain and improve function and some aspects of quality of life in participants with knee OA[7]
. The Osteoarthritis Research Society International (OARSI) recommends the use of walking sticks in the management of knee OA, but states there is a lack of evidence to support their use in multiple joint OA[4]
. EULAR also recommends the use of walking frames or wheeled walkers in patients with OA of the hip or knee[1]
,
[8]
.
There are a number of adaptive measures in the home that lack clinical trial evidence but are rated highly by patients with hip or knee OA
[1]
. These include raising the height of chairs, beds and the toilet seat, or adding handrails to stairs.
EULAR and the American College of Rheumatology (ACR) recommend the use of joint protection in patients with OA affecting their hand[9],[10]
. Joint protection does not mean that the patient should stop using the joint, but should use their hands in a different way to avoid adverse pressure on the affected joint. Pain can act as a warning signal and can indicate that a task involving the use of the hands needs to be performed in a different way.
Patients should not grip things too tightly and should avoid positions that push the joint towards deformity. Arthritis UK (www.arthritisresearchuk.org) provides clear guidance on how to protect the hand joints when performing simple daily activities[11]
. Splints may be used for thumb base OA.
Initial management
There are no pharmacological treatments that are known to prevent or cure OA. Current management is directed at providing symptomatic relief. Pharmacological treatments are adjuncts and the key to management is lifestyle change. It should be ensured that a patient’s continued pain is not being caused by non-adherence to an exercise programme.
Paracetamol or topical non-steroidal anti-inflammatory drugs (NSAIDs) should be used as first-line pharmacological treatment in patients with hand or knee OA. None of the current recognised international guidelines on the management of OA give preference to a particular topical NSAID.
If paracetamol or topical NSAIDs offer insufficient pain relief for people with OA, then the addition of an opioid analgesic should be considered. Evaluation of additional comorbidities, particularly in the elderly, is essential before commencing an opioid analgesic. None of the guidelines give specific dose or timeline recommendations for oral and topical pain relief. An adequate therapeutic trial for a topical NSAID is four weeks, and paracetamol should be trialled on an as required basis before starting a regular treatment regimen.
In a systematic review and a meta-analysis, paracetamol was found to be less effective than originally thought in OA[12],[13]
. Concern has also been raised regarding its safety profile, and that the risk of toxicity associated with paracetamol use is greater than previously perceived at the upper-end of doses, with a dose-related increased incidence of mortality, cardiovascular, gastrointestinal and renal adverse events[14]
[15]
.
Topical NSAIDs are also recommended by both the ACR and EULAR for use in hand and knee OA[2],[9],[10]
. A Cochrane review on the use of topical NSAIDs for chronic musculoskeletal pain in adults concluded that they provide a good level of pain relief in OA[16]
. Topical diclofenac solution was found to be equivalent to oral NSAIDs in knee and hand OA, but there was no evidence for other chronic painful conditions[16]
; diclofenac solutions were found to be more effective than gels.
The Cochrane review found an increase in local adverse events (mild skin reactions) with topical NSAIDs compared with placebo or oral NSAIDs, but no increase in serious adverse events[16]
. Gastrointestinal adverse events with topical NSAIDs did not differ from placebo, but were reduced compared with oral NSAIDs. The review also found that for every six participants treated with the diclofenac solution, only one experienced a good level of pain relief over a period of eight to twelve weeks[16]
. Pain relief with topical NSAIDs may not be instant and is likely to increase over a period of several days; if no benefit is seen after four weeks, treatment should be discontinued.
Paracetamol and topical NSAIDs can be used together, and it may be preferable to use regular paracetamol combined with a topical NSAID before progressing to the next step. This is dependent upon clinical assessment, disease severity, comorbidities and individual patient preference.
NICE recommended management of hand and knee osteoarthritis
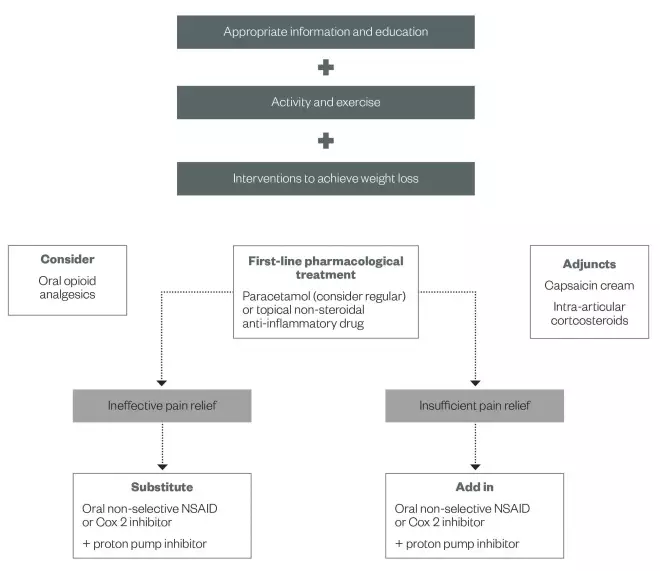
Source: NICE
Oral NSAIDs
NICE recommends evaluating clinical response to paracetamol and/or a topical NSAID before adding additional analgesics. When the initial regimen has provided no pain relief, it should be stopped and an oral NSAID should be tried; this can be either a non-selective NSAID or a cyclo-oxygenase-2 selective (COX 2) inhibitor.
If the initial therapeutic strategy has provided some benefit but there is still insufficient relief of symptoms, then an NSAID (COX 2 inhibitor or non-selective) should be added to the current regimen and the topical NSAID should be discontinued.
The selection of which drug to use should be based on the patient’s additional comorbidities (e.g. age, concomitant medication, cardiovascular disease, renal function, history of peptic ulcer disease) and differing side effect profiles (e.g. gastric, renal and cardiovascular toxicity). The non-selective NSAID or COX 2 inhibitor should be used at its lowest effective dose for the shortest possible period, and patients should be co-prescribed a proton pump inhibitor (PPI) because of the risk of gastrointestinal bleeding.
The use of alternative analgesics (e.g. opioids, or topical capsaicin with regular paracetamol) is recommended by NICE before substituting or adding a non-selective NSAID or COX 2 inhibitor in OA patients taking low-dose aspirin. NICE recommends patients taking any form of NSAID should be prescribed a PPI.
Patients differ in their response to individual NSAIDs. If a patient does not respond to one NSAID they might respond to a different one. Each treatment should be given an adequate therapeutic trial before changing to an alternative; a full analgesic effect should be achieved in one week, while an anti-inflammatory effect may not be achieved for at least three weeks.
Recent studies suggest that some increased cardiovascular risk may apply to all NSAID users, irrespective of their baseline risk, and not only to chronic users[17]
. The greatest concern relates to chronic users of high doses, especially of COX 2 inhibitors and diclofenac[17]
. Naproxen is associated with a lower thrombotic risk than the COX 2 inhibitors. NICE does not support the use of etoricoxib in the management of OA.
Opioid analgesics
The use of opioids is supported in current NICE guidance, but no reference is made to individual drugs[18]
. Opioid analgesics may be indicated for some patients with unacceptable pain despite treatment with oral paracetamol or topical NSAIDs when oral non-selective NSAIDs or COX 2 inhibitors are contraindicated.
Elderly patients may be particularly sensitive to the side effects of opioid analgesics, and concomitant medicines may contribute to the sedative and constipating effects of these medicines. The patient’s risk of falling can also be exacerbated by increased sedation associated with opioid use. Consider co-prescribing appropriate laxatives with opioid analgesics to prevent constipation in at risk patients.
Codeine doses of up to 8mg have not been shown to confer any additional benefit over standard paracetamol. Care should be taken with compound analgesic preparations because, although they may aid adherence, the benefit of low dose codeine 8mg combined with paracetamol has not been substantiated.
A Cochrane review evaluating the benefit of oral or transdermal opioids (excluding tramadol) for osteoarthritis of the knee or hip found a small mean benefit, but a significant increase in adverse effects associated with opioid use[19]
.
Current American College of Rheumatology (ACR) guidance recommends the use of tramadol in OA, but does not support the use of opioids in the management of OA[9]
.
Other treatments
Topical capsaicin cream is recommended by NICE as adjunct therapy for knee and hand OA, and can be considered at any stage of treatment[18]
. Topical capsaicin is not recommended by the ACR for knee OA.
A 2011 comparative efficacy review concluded that topical capsaicin was better than placebo for 50% pain reduction (number needed to treat 8.1)[4]
. The cream is derived from capsicum extract, which comes from the pepper family of plants. Its mechanism of action is not fully understood, but it has been proposed to exert its analgesic effect by depleting and preventing reaccumulation of substance P in peripheral sensory neurones; substance P is thought to be involved in the transfer of pain impulses from the peripheral nervous system to the central nervous system.
A ‘pea-sized’ amount of 0.025% capsaicin cream should be applied to the affected area four times a day for the symptomatic relief of pain. The cream should be gently rubbed in and there should be no residue left on the surface. Application should be spaced evenly throughout waking hours and the dose interval should be more than four hours. It is important the cream is applied at regular intervals to ensure the patient gains tolerance to the burning sensation associated with the initial use of capsaicin.
Patients should be advised that pain relief usually starts within the first week of treatment and increases with continuing regular application for the next two to eight weeks. Patients should be advised to wash their hands immediately after use unless it is being used for hand OA; in this case, the patient should be advised to wait 30 minutes before washing their hands.
Duloxetine is a serotonin and noradrenaline reuptake inhibitor and is recommended by the ACR for some patients with hip OA, and OARSI guidelines state that the use of duloxetine is appropriate in patients with multiple joint OA. The guidelines do not make recommendations as to when duloxetine should be introduced[4],[9]
.
Duloxetine is not licensed for the treatment of OA in the UK. In the United States, the licence for the Cymbalta brand includes the management of chronic musculoskeletal pain. Both a systematic review and a randomised controlled trial comparing duloxetine with placebo found duloxetine to be tolerable and efficacious for chronic pain associated with OA[20],[21]
.
NICE also recommends intra-articular corticosteroid injections as a possible treatment for affected joints. However, it does not recommend that patients are treated with rubefacients; intra-articular hyaluronan injections; glucosamine; or chrondroitin.
There is currently no evidence to support the use of risedronate, strontium ranelate, avocado soybean unsaponifiables and rosehip in the management of OA.
Chondroitin and glucosamine are available for purchase over the counter for pain relief. NICE states that glucosamine and chondroitin products should not be offered for the management of OA[18]
. The ACR also does not support the use of glucosamine or chondroitin in OA of the hip or knee[9]
.
Chondroitin sulphate belongs to a class of very large molecules called glycosaminoglycans (GAGs), which are made up of glucuronic acid and galactosamine. It is manufactured from natural sources such as shark and bovine cartilage. The rationale for taking this supplement in OA is that chondroitin is found endogenously in the cartilaginous tissues of most mammals and serves as a substrate for the formation of the joint matrix structure.
The most recently published meta-analysis showed no statistically significant benefit of chondroitin when compared with placebo[4]
. In a stratified analysis of larger, high quality trials, the effect sizes for pain were small to non-existent[22]
.
Glucosamine is available in three forms: glucosamine hydrochloride, glucosamine sulphate and N-acetyl glucosamine. Glucosamine is required for the synthesis of mucopolysaccharides; these are carbohydrate containing compounds found in tendons, ligaments, cartilage and synovial fluid. A meta-analysis concluded glucosamine does not improve in relation to pain relief in hip or knee OA[23]
. A systematic review also failed to demonstrate any effect on disease modification when compared with placebo at one year follow-up[24]
.
Hyaluronic acid intra-articular injections are not recommended by NICE, but current ACR guidance recommends the use of hyaluronic acid injections in people aged over 74 years with knee OA that is refractory to standard pharmacological treatments[9]
.
Hyaluronan is a natural substance found in the body and is present in high amounts in the synovial fluid of joints. It acts as a lubricant and shock absorber within the joint. Synthesized hyaluronic acid is gel-like in nature and is injected intra-articularly into the knee joint to supplement the natural hyaluronan in the joint and reduce the pain associated with OA of the knee. The injection may reduce pain over a period of one to six months, but there may be an increase in knee inflammation in the short term. Inconsistent conclusions from meta-analysis and conflicting results regarding safety have led to reluctance to support use in the management of knee OA. A recent systematic review found a small but significant effect on knee pain by week four and a peak at week eight (moderate clinical significance); effects lasted up to 24 weeks[4]
.
Disease-modifying anti-rheumatic drugs
(DMARDs) may be of benefit in the management of OA, as imaging techniques have demonstrated that OA is associated with inflammation of the synovium (synovitis). Current guidance does not incorporate the use of DMARDs, but research is being undertaken to evaluate the efficacy of DMARDs in OA.
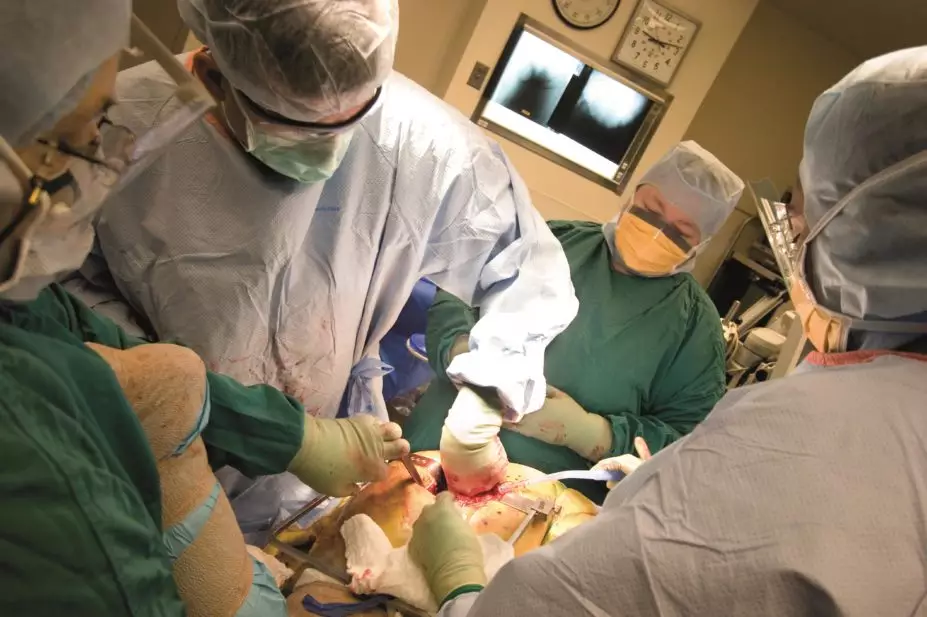
Joint surgery
Joint surgery can be divided into arthroscopic (keyhole) surgery and joint replacement surgery. Arthroscopic surgery is not recommended as part of OA treatment unless a person with knee OA has a clear history of true mechanical locking; this is where the knee suddenly cannot be fully straightened from a position of knee bend. This surgery does not otherwise improve clinical outcomes of knee OA, even with symptoms of ‘giving way’ (typically a symptom of muscle weakness) or evidence of loose bodies on X-rays[8],
[13],
[19],
[20]
.
Joint replacement surgery should be considered when a patient with OA suffers persistent debilitating symptoms despite treatment. Patients with OA can be referred for a surgical opinion irrespective of age, gender, comorbidities, obesity and smoking status. This referral should occur before severe pain or established functional limitation develops.
When considering joint replacement, the benefits and risks of pursuing further medical treatments compared with surgical joint replacement and post-surgical rehabilitation should be discussed.
Tina
Hawkins MRPharmS Ipresc is an advanced clinical pharmacist in rheumatology at Leeds Teaching Hospitals NHS Trust, and Andrew J Barr MBBS MRCP is a clinical research fellow at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds and NIHR Leeds Musculoskeletal Biomedical Research.
The authors have no interests to declare. Andrew Barr’s work is funded by Arthritis Research UK (grant 20154).
References
[1] Fernandes L, Hagen KB, Bijlsma JW et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013; 72(7):1125–1135.
[2] Jordan KM, Arden NK, Doherty M et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62(12):1145–1155.
[3] Bijlsma JW, Berenbaum F & Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. The Lancet 2011;377(9783):2115–2126.
[4] McAlindon TE, Bannuru RR, Sullivan MC et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis and cartilage / OARS. Osteoarthritis Res Soc 2014;22(3):363–388.
[5] Turpin KM, De Vincenzo A, Apps AM et al. Biomechanical and clinical outcomes with shock-absorbing insoles in patients with knee osteoarthritis: immediate effects and changes after 1 month of wear. Arch Phys Med Rehab 2012;93(3):503–508.
[6] Erhart JC, Mundermann A, Elspas B et al. Changes in knee adduction moment, pain, and functionality with a variable-stiffness walking shoe after 6 months. J Orthopaed Res 2010;28(7):873–879.
[7] Jones A, Silva PG, Silva AC et al. Impact of cane use on pain, function, general health and energy expenditure during gait in patients with knee osteoarthritis: a randomised controlled trial. Ann Rheum Dis 2012;71(2):172–179.
[8] Zhang W, Doherty M, Arden N et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005;64(5):669–681.
[9] Hochberg MC, Altman RD, April KT et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care & Res 2012;64(4):465–474.
[10] Zhang W, Doherty M, Leeb BF et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66(3):377–388.
[11] Arthritis Research UK. Protecting your joints.
[12] Towheed TE, Maxwell L, Judd MG et al. Acetaminophen for osteoarthritis. Cochrane Datab Sys Rev 2006(1):CD004257.
[13] Zhang W, Nuki G, Moskowitz RW et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis and cartilage / OARS. Osteoarthritis Research Society. 2010;18(4):476–499.
[14] Craig DGN, Bates CM, Davidson JS et al. Staggered overdose pattern and delay to hospital presentation are associated with adverse outcomes following paracetamol-induced hepatotoxcity. Br J Clin Pharmacol 2012;73(2):285–294. doi:10:1111/j.1365-2125.2011.04067.
[15] Roberts E, Nunes VD & Buckner S. Paracetamol: not as safe as we thought? Ann Rheum Dis 2015;0:1–8
[16] Derry S, Moore RA & Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Datab Sys Rev 2012;9:CD007400.
[17] Trelle S, Reichenbach S, Wandel S et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. The BMJ 2011;342:c7086.
[18] National Institute for Health and Care Excellence. Osteoarthritis: Care and management. London: NICE 2014.
[19] da Costa BR, Nuesch E, Kasteler R et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Datab Sys Rev 2014;9:CD003115.
[20] Citrome L & Weiss-Citrome A. A systematic review of duloxetine for osteoarthritic pain: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Postgraduate Medicine 2012;124(1):83–93.
[21] Frakes EP, Risser RC, Ball TD et al. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Current Med Res Opinion 2011;27(12):2361–2372.
[22] Reichenbach S, Sterchi R, Scherer M et al. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med 2007;146(8):580–590.
[23] Wandel S, Juni P, Tendal B et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. The BMJ 2010;341:c4675.
[24] Lee YH, Woo JH, Choi SJ et al. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int 2010;30(3):357–363.