Microscape / Science Photo Library
Liver disease has become a growing health burden on the NHS, mostly owing to an increase in alcohol consumption, viral hepatitis and obesity. Liver disease is the third major cause of premature death in the UK[1]
; however, the management of patients who are at the advanced (cirrhotic) stage of liver disease remains poor, with only 16% of patients having clear discussions about their preferred place of death and 83% of patients dying in hospital[2]
. This is in part owing to the unpredictable nature of liver disease, precluding early referral for palliative care input. The number of patients with liver disease is expected to increase over the next few years, presenting a challenge for healthcare professionals[1]
.
Patients with end-stage liver disease (ESLD) experience reduced quality of life owing to a variety of symptoms, but with the possibility of alleviation of symptoms following a liver transplant. However, there can be a long waiting time for transplantation, with patients eventually becoming too ill for a major surgical procedure. Palliative care is being increasingly recognised as a way to improve quality of life; patients with ESLD who have received early palliative pharmacological interventions have reported higher quality of life and longer survival[3]
. However, there are many considerations when prescribing drugs in patients with ESLD. This article will discuss the main symptoms that affect quality of life in patients with ESLD and how these can be managed pharmacologically.
Alcohol withdrawal
Assessment
One of the often neglected aspects of inpatient care of alcoholic liver disease patients is the resultant sudden cessation of alcohol as a result of inpatient admission. Up to 20% of inpatients with liver disease are alcohol dependent, 8% of whom will exhibit signs of hyperactivity, tremors, withdrawal seizures and delirium tremens, often necessitating higher dependency care[4]
. Abrupt discontinuation of alcohol results in over-activation of N-methyl-D-aspartate (NMDA glutamate) pathways, down-regulation of gamma-aminobutyric acid (GABA) receptors and increased noradrenergic and dopaminergic transmission[5]
.
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for alcohol withdrawal syndrome largely involves two components:
- A cessation or reduction of previously known prolonged or heavy use of alcohol;
- The development of two or more of the following symptoms:
- Autonomic hyperactivity;
- Worsening tremor;
- Insomnia;
- Vomiting and nausea;
- Hallucinations;
- Psychomotor agitation;
- Anxiety;
- Generalised tonic-clonic seizures[6]
.
Patients with mild (two or three of the symptoms) and moderate (four or five of the symptoms) may be treated in the outpatient setting with sufficient family and social support.
The Clinical Institute Withdrawal Assessment for Alcohol scale, revised version (CIWA-Ar) can then be used to monitor alcohol withdrawal syndrome and to guide pharmacological therapy during the treatment phase[7]
. Scores are based on ten items with a total point system ranging between 0 and 67. However, it is worth noting that CIWA-Ar is reliant upon patient cooperation and communication, and much of the questioning involves subjective responses.
Alternatively, the Severity of Alcohol Dependence Questionnaire can be used as a measure of the severity of dependence. It is divided into five sections, with scores greater than 16 indicating moderate-to-severe alcohol dependence[8]
.
Management
Benzodiazepines can be introduced to patients with moderate-to-severe withdrawal symptoms (CIWA-Ar score ≥15) because they modulate GABA receptors, effectively replacing the inhibitory effects of ethanol. Benzodiazepines have been shown to reduce mortality, duration of withdrawal symptoms[9]
, withdrawal seizures and delirium tremens[10]
. Although there is no single benzodiazepine that has been proven to be more effective than others, consideration should always be given to agents with a rapid onset, longer duration of action to prevent breakthrough symptoms and lesser dependence on hepatic metabolism[9]
. All benzodiazepines are highly protein bound and undergo hepatic metabolisation; chlordiazepoxide and diazepam undergo oxidation producing active metabolites with long half-lives, while oxazepam and lorazepam undergo glucuronidation only with no active metabolites[11]
. In the older population, there is a 50% decrease in clearance and up to a nine-fold increase in terminal half-life of chlordiazepoxide and diazepam[11]
,[12]
. Similarly, patients with ESLD exhibit a similar reduction in clearance and up to a two-fold increase in metabolites from chlordiazepoxide and diazepam use[12]
,[13]
. Lorazepam and oxazepam may be more preferable in patients with ESLD. The choice of benzodiazepine depends on the indication, route availability, and degree of liver and kidney impairment. For patients with delirium tremens, the National Institute for Health and Care Excellence (NICE) guidelines for the diagnosis and management of physical complications of alcohol-use disorders advocate the use of oral lorazepam as first-line treatment, followed by parenteral lorazepam or haloperidol[14]
.
Varices
Management
The incidence of varices occurring in patients with ESLD is 50%, with a third of patients consequently developing variceal bleeds[15],[16]
. Mortality following variceal bleeds can reach 30%, with a significant number of patients requiring admission to the intensive care unit[16]
.
Traditional non-selective beta-blockers (e.g. propranolol) and endoscopic ligation were considered the gold standard in preventing variceal bleeds. Propranolol blocks the adrenergic dilatory tone in mesenteric arterioles, resulting in unopposed alpha-adrenergic mediated vasoconstriction and, therefore, a decrease in portal inflow[17]
. However, since the turn of the 20th century, a growing number of proponents have favoured the use of carvedilol that has demonstrated reductions in hepatic venous pressure gradient measurements in people with ESLD[18],[19]
. In a randomised controlled trial (RCT) comparing carvedilol and band ligation, Tripathi et al. showed that patients who received carvedilol had lower rates of first variceal bleed with no difference in mortality[20]
.
Vasoactive drugs, such as vasopressin and somatostatin (and their respective analogues, terlipressin and octreotide), decrease portal blood flow and can be used to treat variceal haemorrhage. As a group, they have been shown to decrease mortality and improve haemostasis[21]
.
The use of prophylactic antibiotics in patients with variceal bleeds has resulted in an overall reduction in infectious complications, decreased mortality and reduction in the risk of recurrent bleeding, although the choice of antibiotic, duration and group of patients who might benefit remain unclear[22]
.
The use of proton pump inhibitors (PPIs) in cirrhosis has been associated with growing complications and the British Society of Gastroenterology recommends exercising caution with their use[23]
. In an analysis of 1,965 patients, Min et al. demonstrated a greater propensity for the development of spontaneous bacterial peritonitis in patients with cirrhosis and ascites who received PPIs (10.6% and 5.8%; P =0.002)[24]
. PPIs were shown to be the independent risk factor, although this was not confirmed in a larger study published the following year[25]
. A meta-analysis has also shown a 65% increase in Clostridium difficile -associated diarrhoea with PPI use[26]
. In view of conflicting evidence, PPIs should be used with caution. There are insufficient data to support the use of subcutaneous ranitidine (typically given at a dose of 150mg/24 hours) as being more effective than PPIs. Even if the source of bleeding stemmed from ulcers situated around the varices, this could take several weeks to heal, making its use ineffective for a patient with a poor prognosis.
Encephalopathy
Management
Hepatic encephalopathy is defined as an altered mental state secondary to liver failure. The mainstay of treatment aims to reduce ammonia accumulation within the brain. Lactulose remains the preferred choice of laxative with a usual starting dose of around 20–30ml/day, aiming to achieve two or three bowel motions per day. A Cochrane analysis of RCTs found that lactulose was associated with a beneficial effect in the prevention and treatment of hepatic encephalopathy[27]
. In practice, lactulose would be continued when rationalising medicine to provide comfort to the patient. The adverse effects of lactulose are flatulence, bloating and diarrhoea; if these become particularly distressing to the patient, treatment should be reviewed.
An alternative choice is rifaxamin (an antimicrobial agent given to reduce ammonia-producing enteric bacteria[28]
), which is only licensed for recurrent episodes of encephalopathy. Trials have shown rifaxamin to be efficient in treating hepatic encephalopathy, predicting that nine patients would need to be treated for six months to prevent one hospital admission[28]
. Mullen et al. and Bass et al. showed continued reductions in the rate of hepatic encephalopathy and all-cause hospitalisations in patients taking rifaximin[28]
,[29]
. Unfortunately, rifaxamin is only available as a film-coated tablet, making it difficult to administer if a patient has difficulties swallowing.
Benzodiazepines are commonly used in palliative care to treat anxiety. In a survey of UK palliative care physicians, benzodiazepines (93%), usually lorazepam[30]
, were the most frequently prescribed first-line medicines for anxiety in patients with advanced life-limiting disease who had a prognosis of days to weeks. However, for patients with ESLD, they must be used with caution; benzodiazepines can precipitate encephalopathy, making the process more distressing for the patient and relatives.
Muscle cramps
Management
Muscle cramps are a recognised symptom of liver cirrhosis. Although the exact pathology is not fully understood, theories include diuretic induced hypokalaemia (iatrogenic) and vitamin D deficiency. As an inpatient, the mainstay of treatment would be quinine sulphate. Quinine has been used for many years, with a meta-analysis quoting a 21% reduction in cramp frequency and intensity[31]
. However, its use has since been cautioned, with both the Medicines and Healthcare Products Regulatory Agency and the US Food and Drug Administration warning clinicians of associated thrombocytopenia and potential cardiotoxicity. A Cochrane review of 1,586 participants reported only one participant with thrombocytopenia while taking quinine (0.12% risk)[32]
. As a patient nears the end of their life, routine blood monitoring would generally stop and the symptomatic benefit of receiving quinine would far outweigh the risk. If unexplained petechiae (red or purple spots on the skin, caused by a minor bleed from broken capillary blood vessels; see Figure 1), bruising or bleeding does occur, quinine should be stopped.
There is very limited evidence on the use of intravenous albumin to treat muscle cramps. Although the mechanism of action is plausible (i.e. it results in volume expansion and subsequent improvement in hypovolaemia and ischaemia to nerves), only 12 patients were included in the study and it involved intravenous administration weekly for four weeks, rather limiting its feasibility as a therapy[33]
.
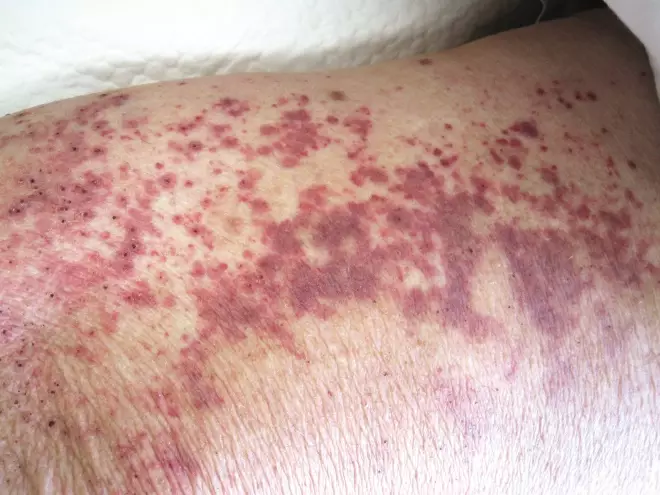
Source: Science Photo Library
Figure 1: Petechiae. Small red or purple spots caused by broken capillary blood vessels are called petechiae. Image shows these spots on a patient’s thigh.
Pain
Management
Non-steroidal anti-inflammatory drugs (NSAIDs) are not used for patients with ESLD because they inhibit the production of prostaglandins that are involved in gastrointestinal mucosal integrity and platelet aggregation. The risk of a bleed would be further increased in those with underlying coagulopathy such as liver cirrhosis. Additionally, the inhibition of prostaglandins is also thought to contribute to a decrease in kidney perfusion. Patients with ESLD require prostaglandins to offset the renin-angiotensin-aldosterone and sympathetic systems that decrease flow to the kidneys[34]
. Stopping prescribed or self-medicated NSAIDs is in line with NICE guidance[35]
.
Morphine has a high first-pass metabolism and a low bioavailability. This oral bioavailability increases in liver disease, making this patient group more susceptible to toxicity. Codeine is converted to its active form of morphine via CYP2D6, which is primarily expressed in the liver, thus its analgesia effect is deemed unreliable. Often it is a patient’s kidney function that determines the choice of opioid. Hepatorenal syndrome is a complication of ESLD, characterised by declining kidney function owing to vasoconstriction. Oxycodone would be the preferred alternative should there be concerns over creatinine clearance as it is shorter acting[36]
. Oxycodone undergoes hepatic metabolism to its active metabolite oxymorphone. Ineffective drug metabolism in liver impairment can lead to a less potent analgesic effect, while decreased clearance and prolonged half-life can result in accumulation[37],[38],[39]
. Both oxycodone and morphine should be used with caution in patients with liver disease, by starting at small doses with prolonged intervals and then titrating doses up as necessary.
Fentanyl patches are not widely used in ESLD patients but had been previously recommended as the opiate of choice. This recommendation was based on a single study where participants had a single dose of fentanyl and their liver impairment at the time was not classified as severe[40]
.
In practice, the aim is to reduce the recommended adult total daily doses of paracetamol from 4g to 3g; however, studies have largely shown that 4g/day is relatively safe in chronic liver disease[41],[42],[43]
. Ethanol is an inducer of CYP2E1, the enzyme responsible for the toxic metabolite N-acetyl-p -benzoquinone imine (NAPQI). Patients who chronically abuse alcohol will have increased levels of NAPQI with the ingestion of paracetamol and, despite evidence to the contrary, it would be wise to remain cautious over the maximum daily dose.
Ascites
Medical management
Ascites, the abnormal accumulation fluid in the abdominal (peritoneal) cavity (see Figure 2), is generally managed based on the presenting symptoms rather than its presence or absence. Broadly speaking, it can be divided into medical and interventional management. First-line therapy would be with a low sodium diet, followed by the use of spironolactone. A typical starting dose would be 100mg per day, increasing to a maximum of 400mg daily[44]
. Unfortunately, mastodynia (breast pain) and gynaecomastia (enlarged male breast tissue) are common side effects of aldosterone use, owing to the reduction in testosterone production, increase in peripheral conversion of testosterone to oestradiol and displacement of oestradiol from sex hormone-binding globulin[45]
. Although a harmless complication, it can be both embarrassing and distressing to patients. Furosemide may be added in refractory ascites up to a maximum of 160mg per day. Furosemide enhances the natriuretic response of an aldosterone antagonist and is therefore not recommended as a monotherapy[44]
.
Interventional management
Patients who do not respond to medical management may require large-volume paracentesis or the insertion of an indwelling peritoneal catheter. An indwelling catheter is a convenient method of allowing ascitic fluid to be removed without the need for repeated needle paracentesis, although the risk of infection is increased. Regular paracentesis is, however, dependent on the availability of local resources. Alternatively, the use of an automated low-flow ascites pump (Alfapump®; Sequana Medical) allows aspiration of fluid in a volume- and time-controlled manner by draining it into the bladder. The cost of the device should be balanced against the cost of lengthy hospital stays and accessibility to personnel to perform frequent paracentesis[46]
.
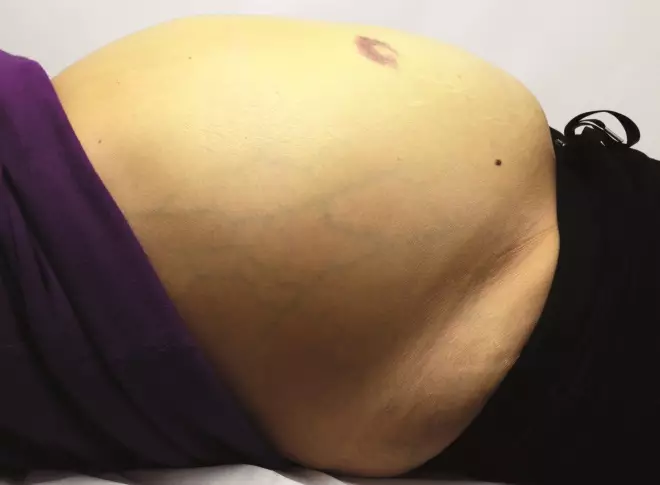
Source: Science Photo Library
Figure 2: Ascites. The abnormal accumulation of fluid in the abdominal cavity (ascites) is managed based on the symptoms rather than its presence or absence
Pruritus
Management
Pruritus can be a distressing symptom for patients with liver disease and can be accompanied with painful skin excoriation (skin picking disorder). Typically, but not exclusively, this is secondary to cholestatic liver disease, which presents owing to a build-up of bile salts in the circulation, manifesting itself through cutaneous skin irritation.
The European Association for the Study of the Liver recommends moisturising and cooling agents (e.g. menthol 1% in aqueous cream) as first-line treatment to sooth itchy and dry skin. Ursodeoxycholic acid is also frequently used in cholestatic liver disease but studies have shown it to be largely ineffective[47]
. Its use is well documented in treatment of pruritus in intrahepatic cholestasis of pregnancy, although evidence is rather sparse for its use outside of this setting. A step-wise approach is sensible when managing pruritus. If simple emollients fail to adequately relief itchy skin, cholestyramine can be used, which works by interrupting the enterohepatic circulation of bile acids. Poor palatability, however, often limits patients’ adherence to therapy. Despite its well-established use, only one underpowered study conducted in 1984 has demonstrated its positive effects[48]
.
Antihistamines (e.g. chlorphenamine, cetirizine, loratadine, fexofenadine and hydroxyzine) have shown limited relief in cholestatic pruritis sufferers[49]
. NICE recommends trialling patients with a sedative antihistamine for two weeks, with treatment being discontinued if no relief has been achieved[50]
. Sedating antihistamines (e.g. chlorphenamine) administered in the evening can aid sleep, preventing nocturnal scratching.
A Cochrane review found rifamipicin and flumecinol to be a superior treatment for pruritus with minimal adverse effects[51]
. Despite its apparent benefits, flumecinol remains unavailable in the UK. Rifamipicin is a potent inducer of CYP450 and interacts with a number of other medicines; as a result, care is needed when initiating a patient on treatment. A meta-analysis of five randomised crossover trials involving 61 patients reported 77% complete or partial resolution of pruritus, at doses ranging from 300mg to 600mg per day[52]
. Although the study only saw four patients suffering from side effects of rifampicin (i.e. nausea, haemolytic anaemia and kidney failure), there are several considerations to make prior to prescribing rifampicin for the alleviation of pruritus. First, rifampicin is excreted via the biliary system and thus the degree of biliary obstruction should be weighed in when considering rifampicin. Second, rifampicin is a potent liver enzyme inducer and has many drug interactions[53]
. Third, high-dose monotherapy has been shown to induce hepatitis[54]
.
Naltrexone has been shown to reduce pruritus compared with placebo but as its primary indication is to block the activity of opioids, its use is generally unsuitable in palliative care. As an opioid-antagonist, it may induce a withdrawal-like reaction, which was first reported in 1988 in all 11 patients who received nalmefene[55]
. In two naltrexone trials, symptoms similar to opiate withdrawal were observed almost consistently, albeit transient, but can be distressing to both patients and medical professionals[56],[57]
. They can be co-administered with clonidine to ameliorate these symptoms[55]
, or started at a low dose and titrated up to achieve a satisfactory therapeutic response. In practice, the first dose is given with caution, and often it is a low dose such as 12.50mg or 6.25mg.
Serotonin reuptake inhibitors (SSRIs) are considered a fourth-line option for cholestatic pruritus because serotonin is thought to induce pruritus by altering itch perception. Studies have shown sertraline to be effective in chronic pruritus caused by liver disease[58]
. Despite paroxetine being used in practice, only a single study with five participants with non-malignant conditions has shown it to be of benefit to pruritic sufferers[59]
. SSRIs can increase bleeding risk in an already haemodynamically unstable population and are extensively metabolised by the liver; thus, careful dosing should be implemented.
Summary
Patients with ESLD will often have frequent hospital admissions, with a high symptom burden. In the UK, the ‘surprise question’, i.e. “would you be surprised if this patient died within the next few months?”, has been suggested to prompt the introduction of end-of-life planning[60]
. Early discussions with patients about treatment expectations could prepare patients and family members for sudden deteriorations in their condition. By reviewing regular medicines and providing symptom relief tailored to patients’ needs, pharmacists and healthcare professionals can ensure that patients remain comfortable during end-of-life care.
Financial and conflicts of interest disclosure:
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was used in the production of this manuscript.
References
[1] Williams R, Aspinall R, Bellis M et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity and viral hepatitis. Lancet 2014;384(9958):1953–1997. doi: 10.1016/S0140-6736(14)61838-9
[2] Low J, Davis S, Vickerstaff V et al. Advanced chronic liver disease in the last year of life: a mixed methods study to understand how care in a specialist liver unit could be improved. BMJ Open 2017;7(8):e016887. doi: 10.1136/bmjopen-2017-016887
[3] Temel JS, Greer JA, Muzikansky A et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678
[4] Carlson RW, Kumar NN, Wong-Mckinstry E et al. Alcohol withdrawal syndrome. Crit Care Clin 2012;28(4):549–585. doi: 10.1016/j.ccc.2012.07.004
[5] McKeon A, Frye MA & Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry 2008;79:854–862. d oi: 10.1136/jnnp.2007.128322
[6] American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Association; 2013
[7] Sulivan JT, Sykora K, Schneiderman J et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x
[8] Stockwell T, Murphy D & Hodgson R. The severity of alcohol dependence questionnaire: Its use, reliability and validity. Br J Addict 1983;78(2):45–156. doi: 10.1111/j.1360-0443.1983.tb05502.x
[9] Mayo-Smith MF, Beecher LH, Fischer TL et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004;164(13):1405. doi: 10.1001/archinte.164.13.1405
[10] Amato L, Minozzi S, Vecchi S & Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev 2010;(3):CD005063. doi: 10.1002/14651858.CD005063.pub3
[11] Saitz R & O’Malley SS. Pharmacotherapies for alcohol abuse: withdrawal and treatment.Med Clin North Am 1997;81(4):881. doi: 10.1016/S0025-7125(05)70554-X
[12] Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996;16(1):49–58. PMID: 8700792
[13] Lejoyeux M, Solomon J & Adès J. Benzodiazepine treatment for alcohol-dependent patient. Alcohol Alcohol 1998;33(6):563–575. PMID: 9872344
[14] National Institute for Health and Care Excellence. Alcohol-use disorders: diagnosis and management of physical complications. Clinical guideline [CG100]. 2010. Available at: https://www.nice.org.uk/guidance/cg100 (accessed October 2018)
[15] Garcia-Tsao G, Sanyal AJ, Grace ND & Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46(3):922–938. doi: 10.1002/hep.21907
[16] O’Brien J, Triantos C & Burroughs AK. Management of varices in patients with cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10(7):402–412. doi: 10.1038/nrgastro.2013.51
[17] D’Amico G, Pagliaro L & Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis 1999;19(4):475–505. doi: 10.1055/s-2007-1007133
[18] Sinagra E, Perricone G, D’Amico M et al. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther 2014;39(6):557–568. doi: 10.1111/apt.12634
[19] Lin HC, Yang YY, Hou MC et al. Acute administration of carvedilol is more effective than propranolol plus isosorbide-5-mononitrate in the reduction of portal pressure in patients with viral cirrhosis. Am J Gastroenterol 2004;99:1953–1958. doi: 10.1111/j.1572-0241.2004.40179.x
[20] Tripathi D, Ferguson JW, Kochar N et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology 2009;50(3):825–833. doi: 10.1002/hep.23045
[21] Wells M, Chande N, Adams P et al. Meta-analysis: vasoactive medicines for the management of acute variceal bleeds. Aliment Pharmacol Ther 2012;35(11):1267–1278. doi: 10.1111/j.1365-2036.2012.05088.x
[22] Hou MC, Lin HC, Liu TT et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology 2004;39(3):746–753. doi: 10.1002/hep.20126
[23] Tripathi D, Stanley AJ, Hayes PC et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64(11):1680–1704. doi: 10.1136/gutjnl-2015-309262
[24] Min YW, Lim KS, Min BH et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther 2014;40(6):695–704. doi: 10.1111/apt.12875
[25] Terg R, Casciato P, Garbe C et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol 2015;62(5):1056–1060. doi: 10.1016/j.jhep.2014.11.036
[26] Janarthanan S, Ditah I, Adler DG & Ehrinpreis MN. Clostridium difficile -associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–1010. doi: 10.1038/ajg.2012.179
[27] Gluud LL, Vilstrup H & Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database of Syst Rev 2016;4:CD003044. doi: 10.1002/14651858.CD003044.pub3
[28] Mullen KD, Sanyal AJ, Bass NM et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 2014;12(8):1390–1397.e2. doi: 10.1016/j.cgh.2013.12.021
[29] Bass NM, Mullen KD, Sanyal Aet al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362(12):1071–1081. doi: 10.1056/NEJMoa0907893
[30] Atkin N, Vickerstaff V & Candy B. ‘Worried to death’: the assessment and management of anxiety in patients with advanced life-limiting disease, a national survey of palliative medicine physicians. BMC Palliat Care 2017;16(1):69. doi: 10.1186/s12904-017-0245-5
[31] Man-Son-Hing M, Wells G & Lau A. Quinine for nocturnal leg cramps: a meta-analysis including unpublished data. J Gen Intern Med 1998;13(9):600–606. doi: 10.1046/j.1525-1497.1998.00182.x
[32] El-Tawil S, Al Musa T, Valli H et al. Quinine for muscle cramps. Cochrane Database Syst Rev 2015;(4):CD005044. doi: 10.1002/14651858.CD005044.pub3
[33] Angeli P, Albino G, Carraro P et al. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology 1996;23(2):264–273. doi: 10.1002/hep.510230211
[34] Laffi G, La Villa G, Pinzani M et al. Arachidonic acid derivatives and renal function in liver cirrhosis. Semin Nephrol 1997;17(6):530–548. PMID: 9353864
[35] National Institute for Health and Care Excellence. Acute upper gastrointestinal bleeding in over 16s: management. Clinical guideline [CG141]. 2016. Available at: https://www.nice.org.uk/guidance/cg141 (accessed October 2018)
[36] Murphy EJ. Acute pain management pharmacology for the patient with concurrent renal or hepatic disease. Anaesth Intensive Care 2005;33(3):311–322. PMID: 15973913
[37] Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol 2008;64(12):1147–1161. doi: 10.1007/s00228-008-0553-z
[38] Bosilkovska M, Walder B, Besson M et al. Analgesics in patients with hepatic impairment: pharmacology and clinical implications.Drugs 2012;72(12):1645–1669. doi: 10.2165/11635500-000000000-00000
[39] Chandok N & Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010;85(5):451–458. doi: 10.4065/mcp.2009.0534
[40] Haberer JP, Schoeffler P, Couderc E & Duvaldestin P. Fentanyl pharmacokinetics in anaesthetized patients with cirrhosis. Br J Anaesth 1982;54(12):1267–1270. doi: 10.1093/bja/54.12.1267
[41] Benson GD. Acetaminophen in chronic liver disease. Clin Pharmacol Ther 1983;33(1):95–101. doi: 10.1038/clpt.1983.14
[42] Zimmerman HJ & Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22(3):767–773. doi: 10.1016/0270-9139(95)90295-3
[43] Kuffner EK, Dart RC, Bogdan GM et al. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Intern Med 2001;161(18):2247–2252. doi: 10.1001/archinte.161.18.2247
[44] European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53(3):397–417. doi: 10.1016/j.jhep.2010.05.004
[45] Haynes BA & Mookadam F. Male gynecomastia.Mayo Clin Proc 2009;84(8):672. PMID: 19648382
[46] Stirnimann G, Banz V, Storni F & De Gottardi A. Automated low-flow ascites pump for the treatment of cirrhotic patients with refractory ascites. Therap Adv Gastroenterol 2017;10(2):283–292. doi: 10.1177/1756283X16684688
[47] Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med 1997;336(10):691–695. doi: 10.1056/NEJM199703063361003
[48] Duncan JS, Kennedy HJ & Triger DR. Treatment of pruritus due to chronic obstructive liver disease. Br Med J (Clin Res Ed) 1984;289(6436):22. PMID: 6145483
[49] Levy C. Management of pruritus in patients with cholestatic liver disease. Gastroenterol Hepatol (NY) 2011;7(9):615–617. PMID: 22299001
[50] National Institute for Health and Care Excellence. Itch — widespread. 2015. Available at: https://cks.nice.org.uk/itch-widespread (accessed October 2018)
[51] Siemens W, Xander C, Meerpohl JJ et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database of Syst Rev 2016;11. doi: 10.1002/14651858.CD008320.pub3
[52] Khurana S & Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int 2006;26(8):943–948. doi: 10.1111/j.1478-3231.2006.01326.x
[53] Gorski JC, Vannaprasaht S, Hamman MA et al. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther 2003;74(3):275–287. doi: 10.1016/S0009-9236(03)00187-5
[54] Prince MI, Burt AD & Jones DE. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut 2002;50(3):436–439. PMID: 11839728
[55] Thornton JR & Losowsky MS. Opioid peptides and primary biliary cirrhosis. Br Med J 1988;297:1501–1504. doi: 10.1136/bmj.297.6662.1501
[56] Wolfhagen FHJ, Sternieri E, Hop WCJ et al. Oral naltrexone for cholestatic pruritus: a doubleâ€blind, placeboâ€controlled study. Gastroenterol 1997;113(4):1264–1269. doi: 10.1053/gast.1997.v113.pm9322521
[57] Terg R, Coronel E, Sordá J et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. Hepatology 2012;37(6):717–722. doi: 10.1016/S0168-8278(02)00318-5
[58] Mayo MJ, Handem I, Saldana S et al. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology 2007;45(3):666–674. doi: 10.1002/hep.21553
[59] Zylicz Z, Krajnik M, Sorge AA & Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage 2003;26(6):1105–1112. doi: 10.1016/j.jpainsymman.2003.05.004
[60] Downar J, Goldman R, Pinto R et al. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ 2017;189(13):E484–E493. doi: 10.1503/cmaj.160775