Abstract
Liver disease is the third biggest cause of premature mortality in the UK. Community pharmacy can effectively identify people with hepatitis C but is not routinely used to identify people with alcohol-related liver disease.
Some community pharmacists offer alcohol screening and a brief intervention to reduce alcohol misuse, and evidence has shown that pharmacists can identify a high proportion of people who drink above recommended limits when compared to the general population. A unique attribute of community pharmacies is their accessibility to those from areas of higher deprivation. It is well recognised that alcohol-related mortality is highest in these areas.
The ability to identify people who drink alcohol above recommended limits, combined with its accessibility to the populations most at risk of alcohol harm, shows community pharmacy has clear potential to identify people who may have undiagnosed alcohol-related liver disease. However, research is required to optimise and establish how community pharmacists can effectively work in partnership with medical services to ensure these patients receive appropriate ongoing care.
Key words: community pharmacy; alcohol; liver disease; cirrhosis; risk; testing; diagnosis.
Key points
- Deaths resulting from alcohol-related liver disease (ArLD) continue to rise;
- Identifying early-stage ArLD can reduce morbidity and mortality by motivating reduction in alcohol intake and preventing complications;
- Community pharmacists can identify people at risk of ArLD;
- Linking people identified by community pharmacists as at risk of ArLD with a test for liver fibrosis could increase the detection of undiagnosed ArLD.
Introduction
The role of community pharmacy in improving public health has continued to expand, as envisaged in the 2008 white paper ‘Pharmacy in England — Building on strengths, delivering the future’[1]. The Healthy Living Pharmacy (HLP) Framework, created to increase community pharmacy delivery of a broad range of public health services, demonstrates this potential[2].
Community pharmacy is a vital part of the ‘NHS long-term plan’[3]. In keeping with this, the ‘Community pharmacy contractual framework’ (CPCF) for 2019/2020 to 2023/2024 requires all community pharmacies to meet HLP level 1 requirements from 1 January 2021 and comments on a “fundamental shift towards clinical service delivery focused initially on minor illness and the prevention and detection of ill health”[4,5].
The capability of community pharmacy to prevent and detect ill-health is enhanced by the fact that community pharmacists are one of the most accessible healthcare professionals[6]. Their position in the community also means they can address inequalities in access to healthcare. For example, the number of GPs per 100,000 of the population is lower in areas of higher deprivation but, conversely, access to community pharmacies is greatest in areas of highest deprivation — the so-called ‘Positive Pharmacy Care Law’[7,8].
This accessibility, combined with the fact that around 90% of the UK population visit a community pharmacy at least once per year, makes community pharmacies a strategic place to undertake population screening for disease[9]. This potential has been studied in many disease areas, including diabetes, cardiovascular disease, respiratory disease, colorectal cancer, and osteoporosis, and there appears to be high participant satisfaction and acceptability for these services[6,9–12]. Researchers have highlighted that higher quality research is needed to establish effectiveness of these services, but some recent studies have already demonstrated their efficacy and cost-effectiveness[6,9,10].
Support for case finding in community pharmacy is shown by the allocation of funding for hepatitis C (HCV) testing in the new CPCF[5]. The service offers a HCV antibody test to individuals who inject drugs who are not currently accessing treatment services. HCV antibody testing in community pharmacy, based on any risk factor (including injectable drug use), has been shown to be cost effective and demonstrates that community pharmacists are capable of reaching individuals who are not engaged with other services[13–15]. Other research has also shown the ability of community pharmacists to not only test for HCV but provide HCV treatment, resulting in better treatment outcomes compared to conventional care[16,17].
Alcohol and liver disease
The expansion of HCV testing into community pharmacy comes amid greater attention to the mortality from liver disease, which increased 400% in the UK between 1970 and 2015[18]. Liver disease represents the third largest cause of premature death in the UK, accounting for around 12,000 deaths per year, with only ischaemic heart disease and suicide ranking higher[18]. In 2013, the Department of Health listed liver disease as one of the ‘five big killer diseases’ in the Living Well for Longer Campaign, alongside cancer and stroke[19]. In the same year, the Lancet Commission into liver disease was created with the aim of providing the strongest evidence-based recommendations to reduce the premature mortality and disease burden from avoidable causes of liver disease. Its first recommendation was “improving expertise and facilities in primary care to strengthen detection of early disease and its treatment, and screening of high-risk patients in the community”[18,20,21]. Additionally, improving community-based care for patients with alcohol-related liver disease (ArLD) was a research priority set by the James Lind Alliance, a non-profit initiative established in 2014 and funded by the National Institute for Health Research, which identifies areas of evidence uncertainty by bringing together patients, carers, and health and social care professionals[22].
HCV testing in community pharmacy is one response to these recommendations; however, the majority of premature mortality from liver disease is associated with ArLD[18]. A 2016 report from the Office for National Statistics highlighted that almost 90% of alcohol-related deaths in England and Wales are caused by ArLD[23]. The consequences of alcohol misuse go beyond liver disease; in England, alcohol misuse is the biggest risk factor for early mortality, ill health and disability in people aged 15–49 years[24]. And, in 2018, data show that 180,000 working years were lost to alcohol misuse[25]. Alcohol is also a factor in health inequality — the most deprived areas have the greatest rates of alcohol-related harm, including ArLD[18]. Alcohol misuse and ArLD have been of particular relevance during the ongoing COVID-19 pandemic, during which there has been a reported increase in alcohol use and an associated rise in alcohol-related hospital admissions in the UK[26,27].
There are many locally-commissioned community pharmacy alcohol screening and brief intervention services[28]. Alcohol brief interventions are short, empathetic and structured conversations that aim to motivate and support individuals to think about and/or plan a change in their drinking behaviour[29]. These community pharmacy services aim to address the problem of alcohol misuse but the role of community pharmacy in going further to identify ArLD has not been considered.
This article describes current practice for identifying ArLD in the community and aspects that have been conducted in community pharmacy. It then considers how a care pathway to identify ArLD could be deployed in a community pharmacy setting.
Identifying alcohol misuse
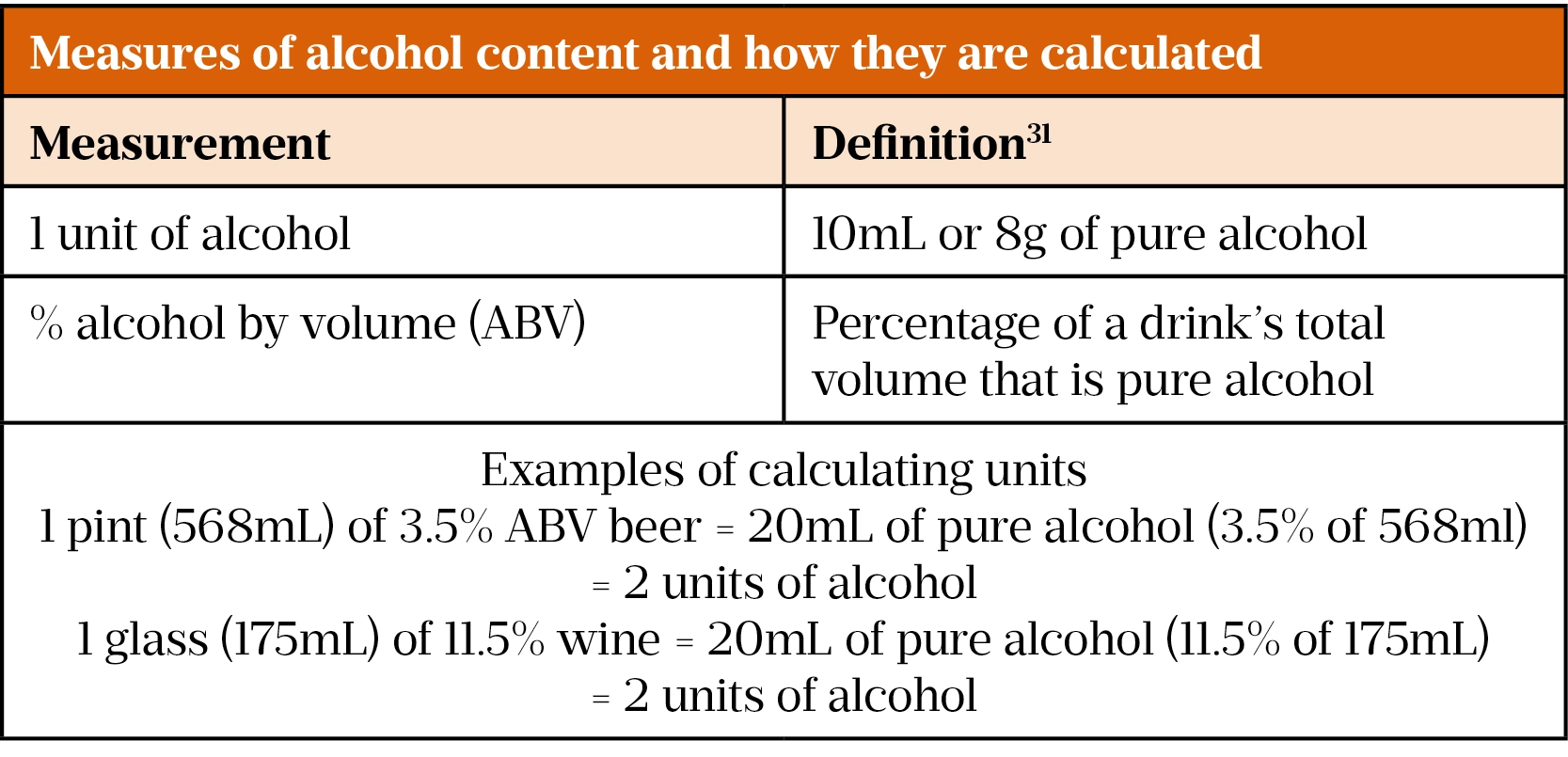
To identify ArLD, alcohol misuse must be identified. Current UK guidance advises a limit of 14 units of alcohol per week for men and women to keep health risks from alcohol to a low level[30]. In the UK, the alcohol content of a drink is shown as ‘alcohol by volume’ (ABV). Table 1 shows the definitions of a unit of alcohol and ABV and shows how these are used to calculate the alcohol content of a drink[31].
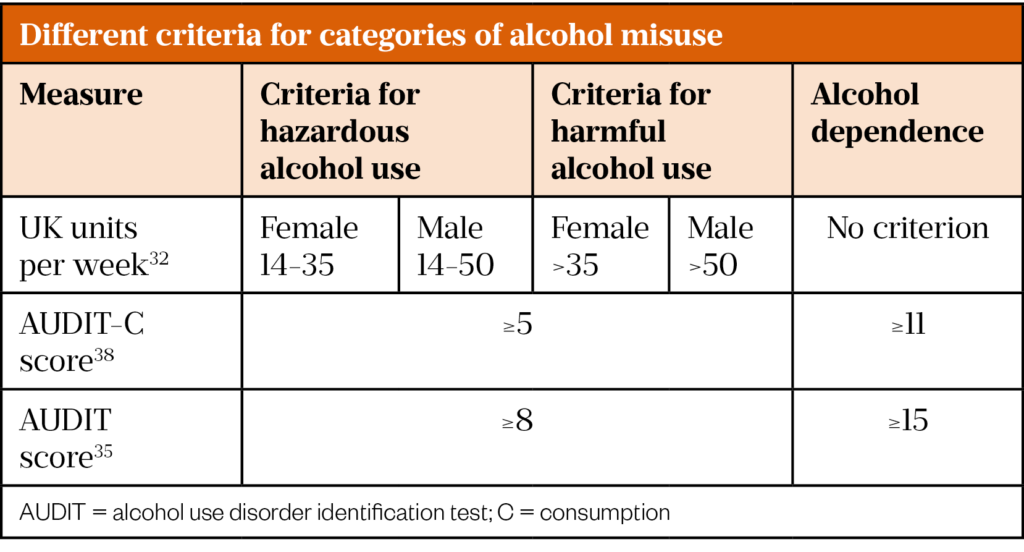
Consuming alcohol above recommended limits constitutes alcohol misuse and can be termed hazardous or harmful[32]. The World Health Organization (WHO) defines hazardous alcohol use as that which increases the risk of harmful health consequences. Harmful alcohol use is defined as that which has caused damage to health[33]. In the UK, the National Institute for Health and Care Excellence (NICE) also provides weekly consumption-based definitions (see Table 2).
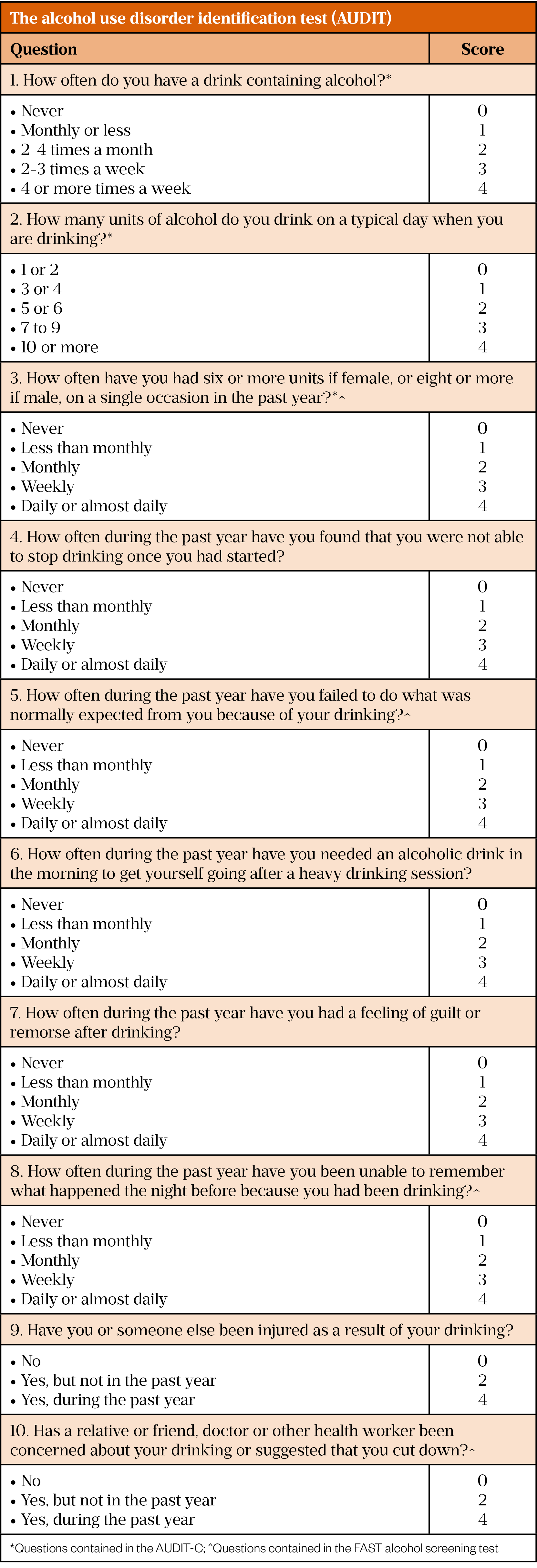
Alongside these definitions is the concept of alcohol use disorder (AUD), which puts greater focus on aspects of dependence. AUD is defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) as “a problematic pattern of alcohol use leading to clinically significant impairment or distress”[34]. The WHO-approved Alcohol Use Disorder Identification Test (AUDIT) is regarded as the ‘gold standard’ alcohol use screening test and is the most widely used tool in primary care[27,35,36]. It contains ten multiple choice questions that enquire about a person’s alcohol intake, potential dependence on alcohol and experience of alcohol-related harm[35]. Each question is scored individually and the sum of the ten answers gives the total AUDIT score, ranging from 0 to 40. The AUDIT questions and how they are scored are shown in Figure 1. The AUDIT-Consumption (AUDIT-C) score is an abbreviated version of the AUDIT that uses the first three questions of the AUDIT score, which ask about alcohol intake. The Fast Alcohol Screening Test (FAST) is another alcohol use screening test that asks four questions taken from the AUDIT (see Figure 1). A FAST score of ≥3 is deemed ‘positive’ and should then prompt completion of the remaining AUDIT questions[37].
Both the AUDIT and AUDIT-C scores can be used to identify hazardous or harmful alcohol use and alcohol dependence as shown in Table 2[38].
What is alcohol-related liver disease?
The term ‘alcohol-related liver disease’ encompasses a spectrum of liver disease caused by alcohol misuse. According to European guidelines, criteria for a diagnosis of ArLD include patients with clinical and/or biological abnormalities suggestive of liver injury — such as fatty liver on imaging, abnormal liver blood tests, evidence of liver fibrosis — who have a regular alcohol intake of >20g/day in women (17.5 UK units per week) and >30g/day in men (26 UK units per week)[36]. The spectrum of ArLD begins with liver steatosis (i.e. fatty liver), which is present in almost all heavy drinkers[39]. In a subset of patients who continue to drink excess alcohol, there is progression to liver inflammation and injury, which is termed ‘steatohepatitis’.
Steatohepatitis will continue with ongoing heavy alcohol intake and may result in further progression to liver fibrosis and, ultimately, cirrhosis, which is the final and irreversible stage of fibrosis[40]. The development of liver fibrosis in ArLD indicates those with progressive disease that may require specialist input, but it is generally asymptomatic until advanced and irreversible[40,41]. This means that diagnosis of ArLD at this late stage is common: nearly half of all patients with liver cirrhosis are first diagnosed during an emergency hospital admission and alcohol is the most common cause of cirrhosis in these patients (other causes of cirrhosis include hepatitis, non-alcoholic fatty liver disease and bile duct disorders)[42]. The asymptomatic development of liver fibrosis means routine testing is an important component in the diagnosis of suspected ArLD[40].
Identification of ArLD at an earlier stage can drive behaviour change to reduce alcohol consumption, which in turn can prevent progression to cirrhosis[43,44]. Earlier diagnosis of cirrhosis improves prognosis by allowing for the motivation for alcohol abstinence and facilitating engagement with interventions to prevent and/or treat complications of cirrhosis (e.g. liver cancer)[45,46]. Strategies for reduced intake and abstinence from alcohol are beyond the scope of this article but national guidance and advice on application in community pharmacy has been produced[27,47]. Importantly, it is recognised that brief alcohol interventions in primary health care (including community pharmacy) are effective in reducing alcohol-related problems[48].
Testing for liver fibrosis
Historically, a liver biopsy has been the only way to identify liver fibrosis[49]. Liver biopsy remains the gold standard for both diagnosis and staging of ArLD but is an invasive test with associated morbidity and so is not routinely recommended[36]. The risk of liver biopsy has led to the development of non-invasive liver fibrosis tests (NILTs), which are now part of routine practice in the UK[41].
There are several types of NILTs, including blood or imaging tests[50]. Current UK guidance advises the use of the enhanced liver fibrosis (ELF) blood test or transient elastography (TE [a form of ultrasound scan]) in patients at risk of ArLD[41,51]. An ELF test requires a standard blood sample, similar to other blood tests routinely performed in the community. TE requires a specialist device but is an easy to learn procedure, requiring only minimal training and providing a result at the point of care[50]. It has widespread use in secondary care but is increasingly being performed in the community (usually by a trained nurse) via primary care outreach services and in a few locally funded primary care centres[52]. Both tests give a numerical result that is compared against recognised cut-off values to determine whether there is suspected significant liver fibrosis.
Existing services that identify alcohol-related liver disease in the community
NICE and the British Society of Gastroenterology advises testing for liver fibrosis in individuals at risk of ArLD, and the NHS Health Check ‘Best practice guidance’, published in October 2019, advises referring anyone with an AUDIT score of ≥16 for a liver fibrosis test[41,51,53].
In addition to national guidance, many NHS trusts have developed primary care pathways to assist GPs in appropriate investigation and referral of liver disease[54–57]. Entry into such pathways may be from blood test and imaging abnormalities, or based on risk factors alone (e.g. the presence of alcohol misuse). In advanced liver disease, liver blood tests may be within normal range, demonstrating the importance of identifying risk factors[41].
Primary care pathways incorporate one or more NILTs that are performed in the community. NILT cut-off values are provided to inform primary care clinicians which patients may have significant fibrosis, and should therefore be referred onto secondary care, as well as patients that can be monitored in the community[54–56]. This approach has been shown to be cost effective[58]. An example pathway is shown in Figure 2.
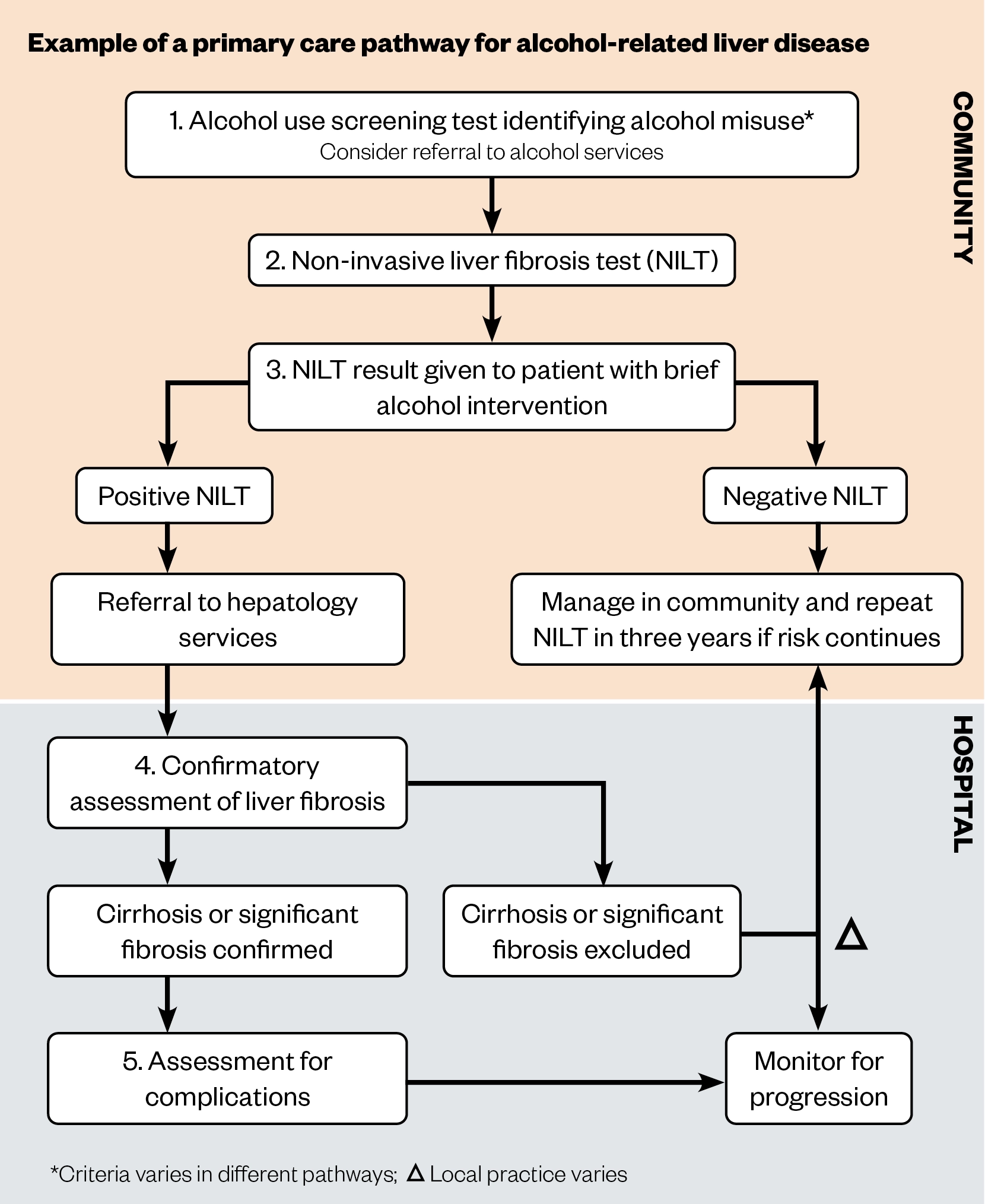
Existing primary care pathways are not without limitations. Identification of alcohol misuse is the universal component of these services, but has been shown to be suboptimal in general practice. A cross-sectional study published in 2019 by Mansfield et al. demonstrated that only 48.8% of 1.5 million registered patients had a record of their alcohol consumption in the past five years[59]. In addition, a study that used GP electronic records to identify risk factors for liver disease found the prevalence of hazardous alcohol use was 6.3%, much less than the estimated general population prevalence of 22%[60,61]. This may be explained by suboptimal recording of alcohol consumption but could also indicate that individuals with hazardous alcohol use do not consult their GP or are not truthful when queried. Additionally, 15% of patients with liver cirrhosis are aged younger than 45 years at diagnosis, with ArLD the leading aetiology[62]. NHS Health Checks are only offered every five years to those aged 40–74 years, therefore opportunities for earlier diagnosis can be missed in those aged under 45 years[53].
Evidence for community pharmacists identifying those at risk of alcohol-related liver disease
Individual studies have demonstrated community pharmacists’ ability to perform alcohol use screening tests to identify people who are misusing alcohol. Health Survey for England data show that 22% of adults in England drink hazardous amounts of alcohol and 4% drink harmful amounts[61]. Published community pharmacy studies involving alcohol use screening tests show a higher proportion of hazardous alcohol use, ranging from 27% to as high as 79%, as shown in Table 3[63–73]. The ability to identify a higher prevalence of hazardous alcohol use would make community pharmacy a strategic place to identify ArLD.
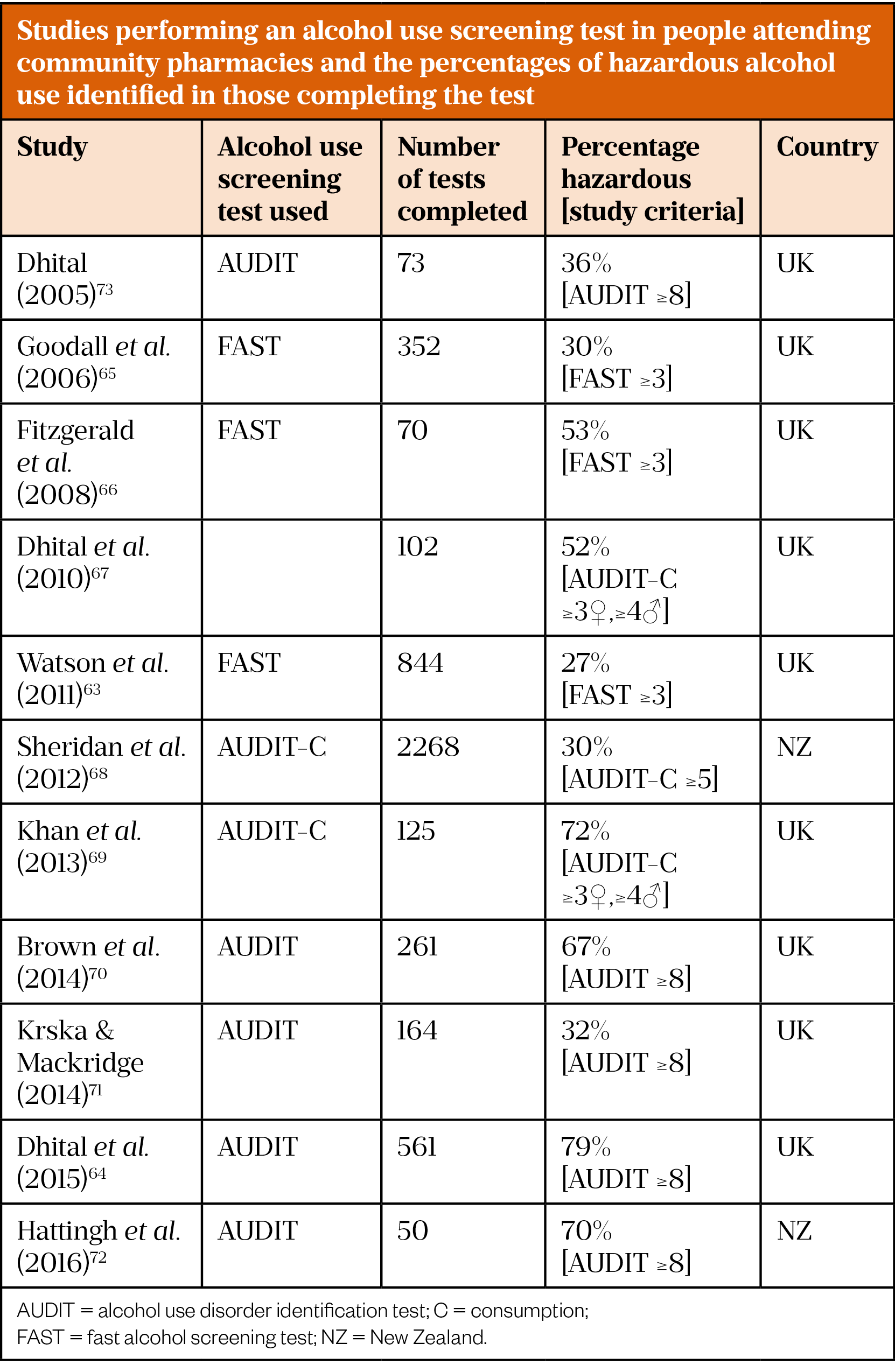
Most of the studies in Table 3 offered an alcohol use screening test to any adult attending the community pharmacy, while five of the studies used a case-finding strategy by offering the test to targeted groups[63,64,69,70,72]. Four of these five studies identified the highest percentages of hazardous alcohol use [64,69,70,72]. Brown et al. only offered the test to women who were accessing the community pharmacy for emergency hormonal contraception(70). The other three studies offered tests to people requesting treatment for predefined symptoms that may be related to alcohol use (e.g. reflux, poor sleep). Additionally, the presence of behaviours including use of certain medication prescriptions, use of smoking cessation services and asking for alcohol advice were used to prompt the offer of a test[64,69,72]. The acceptability of routine alcohol use screening by community pharmacists as part of medication reviews has been recently demonstrated in the results of a randomised control trial (RCT) published in 2020 by Stewart et al.[74].
The studies outlined in Table 3 demonstrate the ability of community pharmacists to identify people who are drinking alcohol at potentially harmful levels and who may benefit from an assessment for ArLD.
Evidence for a pharmacy-based brief intervention to reduce risk of alcohol-related liver disease
In 2015, Dhital et al. assessed whether a brief alcohol intervention delivered by community pharmacists in comparison with a leaflet control was effective in reducing hazardous and harmful alcohol use at three months, determined by change in AUDIT score[64]. All participants required an AUDIT score of between 8 and 19 to be eligible for the study. Out of 561 customers who were tested, 407 were eligible and participated. The study did not find any significant difference in AUDIT score between the intervention and control groups, and AUDIT score did not significantly change from baseline to follow up in either group. However, a secondary outcome examining AUDIT-C scores showed statistically significant reductions in mean AUDIT-C score of 0.75 (95% CI 0.41–1.08) in the intervention group and 0.69 (95% CI 0.35–1.03) in the control group, indicating a decrease in alcohol consumption[64].
A reduction in alcohol consumption has been seen in other pharmacy-based studies. In 2013, Khan et al. followed up 41 hazardous drinkers three months after a pharmacy-delivered alcohol brief intervention and found a statistically significant decrease in the median number of drinking days per week from three to one and an 84% reduction in the number of alcohol units consumed[69].
Then, in 2016, Hattingh et al. followed up ten participants after a pharmacy-delivered alcohol screening and brief intervention and observed three of the five participants with hazardous or harmful alcohol use had reduced their level of drinking at one month follow up[73].
This trend of reduction in alcohol consumption is in keeping with the known effectiveness of alcohol screening and brief intervention in primary care populations[48]. Importantly, it may signal an ability for community pharmacists to reduce risk of ArLD and thereby demonstrate the further utility of community pharmacy in ArLD pathways.
What could a community pharmacy pathway for identifying alcohol-related liver disease look like?
Current national guidance advises the offer of a NILT to people at risk of ArLD, highlighting the potential for an ArLD identification pathway in community pharmacy; however, there are currently no existing ArLD pathways in community pharmacy. Community pharmacy HCV antibody testing is now a CPCF-commissioned service[75]; the testing pathway in this service could inform the structure of a community pharmacy ArLD identification pathway. In the HCV antibody testing service, those offered testing are consented pre-test for onward referral (if their test is positive) to the local operational delivery network (a central hub that coordinates patient pathways between healthcare providers) for further testing and treatment[75]. By combining this model with the structure of existing community ArLD pathways (see Figure 2), we present a conceptual model of what a community pharmacy ArLD pathway could look like (see Figure 3).
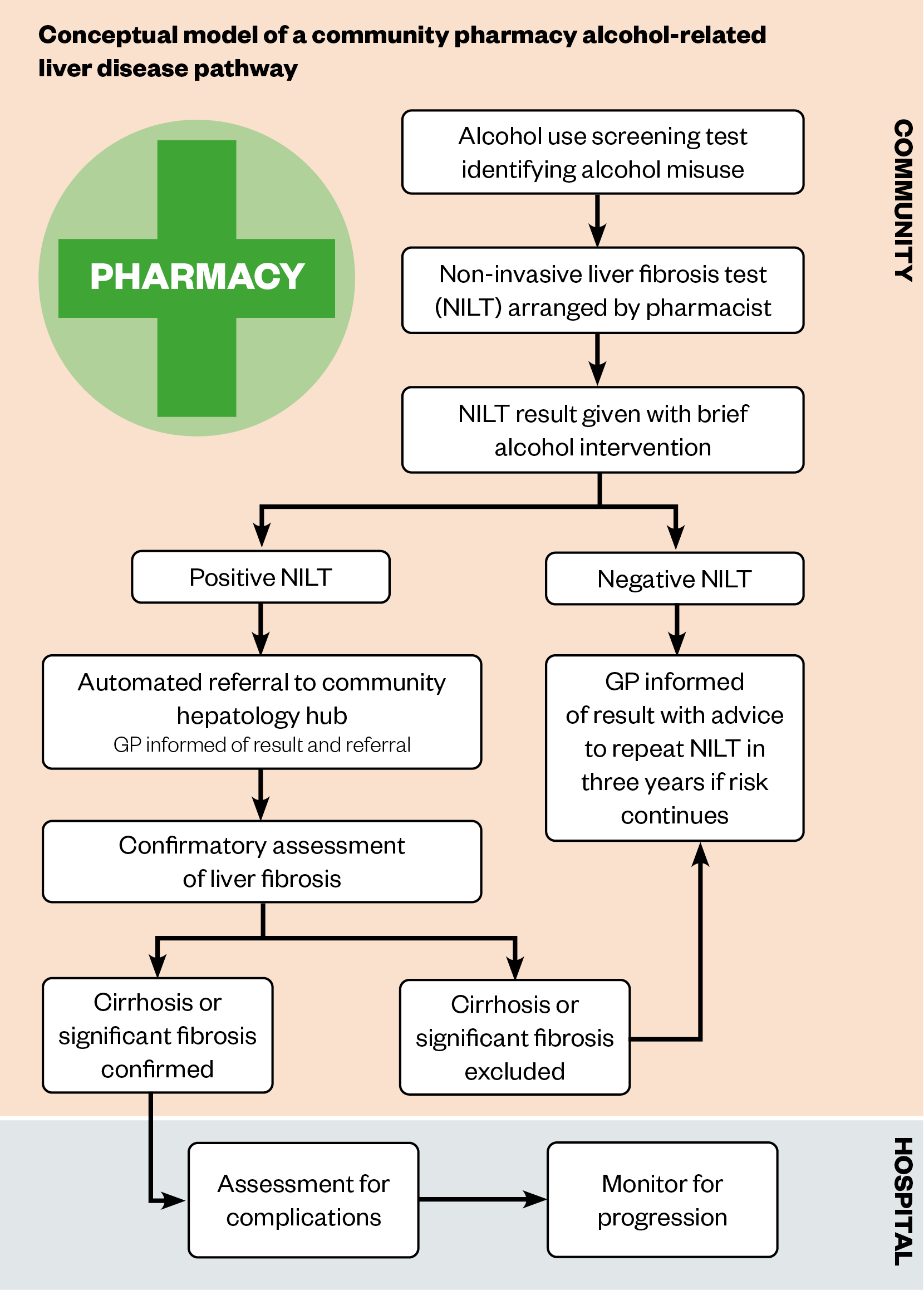
For such a pathway to be developed and implemented, research is needed to establish its design, acceptability and feasibility. This initial exploratory work would have to consider existing literature that describes barriers to pharmacy disease screening and case finding services[6,9]. Known barriers include patient uptake of onward referral for confirmatory testing/ongoing care, pharmacist knowledge and confidence, public and other healthcare providers’ perception of pharmacist competency, a perceived lack of privacy and confidentiality in the pharmacy environment, and time pressures of pharmacy staff; the latter related to inadequate funding preventing the employment of sufficient staff[6,9].
A challenge when considering an ArLD pathway in community pharmacy is the NILT. A key component of the HCV antibody testing service is the use of a point-of-care test, performed by the pharmacist[75]. TE is the only recommended point-of-care NILT in ArLD but, to our knowledge, has never been used in the community pharmacy setting. The alternative to TE is a blood sample NILT, such as the nationally recommended ELF test, but there are no point-of-care blood-based NILTs[41]. Encouragingly the ability of community pharmacists to arrange a blood sample for NILTs and interpret the results has been recently demonstrated by Radley et al. in their study of a pharmacy-based HCV treatment service, where a nurse or phlebotomist attended pharmacies to take the required blood sample[16]. Whether this could be emulated in a community pharmacy ArLD care pathway or whether TE could/should be the NILT are some of the questions that research into a community pharmacy ArLD pathway would need to address. The Box below details some research questions that must be addressed for the implementation of the aforementioned pathway.
Box: A sample of research questions that need to be addressed in order to design an effective community pharmacy-based care pathway for alcohol related liver disease
- Which non-invasive liver test (NILT) for liver fibrosis should be done?
- Can the NILT be performed in community pharmacy and if so who should do it?
- How best is the NILT result given and who should deliver this?
- What training and support for community pharmacists would be needed?
- Should onward referral be direct to hepatology services or via general practice?
- What could be the impact of the potential increased workload on general practice and/or hepatology services?
- How can alcohol addiction services be incorporated to maximise public health benefits of the service?
Summary
This article outlines evidence showing that community pharmacy could play an important role in the community care pathway for people at risk of ArLD. Alcohol misuse causes three-quarters of deaths from liver disease in the UK[18]. Deprived areas have the highest burden of ArLD and alcohol-related harm, but also have the greatest access to community pharmacy services[8,18]. Expanding community pharmacy services therefore presents an opportunity to address the health inequality of alcohol-related death. Earlier diagnosis of ArLD saves lives through motivating reduced alcohol consumption and facilitating engagement with interventions to prevent complications[45,46,76]. Existing care pathways for ArLD in community settings aid earlier diagnosis but they are not without limitations[77].
There is substantial evidence that individuals with alcohol misuse can be identified through simple alcohol use screening tests performed in community pharmacy. There is also evidence that alcohol use screening tests performed in community pharmacy identify a greater proportion of people with alcohol misuse than is present in the general population. While it is uncertain whether a pharmacy delivered alcohol brief intervention leads to a reduction in alcohol consumption, community pharmacy could play an important role in linking at-risk patients directly to tests for ArLD. Additional research is needed to examine the structure, feasibility and effectiveness of a community pharmacy-based care pathway for ArLD.
Financial and conflicts of interest disclosure
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was used in the production of this manuscript.
- 1Pharmacy in England Building on strengths-delivering the future. Department of Health. 2008.https://www.gov.uk/government/publications/pharmacy-in-england-building-on-strengths-delivering-the-future (accessed Sep 2021).
- 2Duggan C, Evans D, Holden M, et al. Evaluation of the Health Living Pharmacy Work Programme 2011-2012. Pharmaceutical Services Negotiating Committee. 2013.https://psnc.org.uk/sheffieldlpc/wp-content/uploads/sites/94/2013/06/Evaluation_of_HLP_pathfinder_work_programme-FINAL.pdf (accessed Sep 2021).
- 3Community pharmacy at the heart of primary care. Pharmaceutical Journal Published Online First: 2019. doi:10.1211/pj.2019.20207379
- 4PSNC Briefing 042/20 : How to become a Healthy Living Pharmacy (HLP) and maintain that status. Pharmaceutical Services Negotiating Committee. 2020.https://psnc.org.uk/wp-content/uploads/2020/11/PSNC-Briefing-042.20-How-to-become-a-Healthy-Living-Pharmacy-HLP-and-maintain-that-status-updated-November-2020.pdf (accessed Sep 2021).
- 5The Community Pharmacy Contractual Framework for 2019/20 to 2023/24: supporting delivery for the NHS Long Term Plan. Department of Health and Social Care. 2019.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/819601/cpcf-2019-to-2024.pdf (accessed Sep 2021).
- 6Community pharmacists’ contribution to public health: assessing the global evidence base. Clinical Pharmacist Published Online First: 2018. doi:10.1211/cp.2018.20204556
- 7Dayan M. “Are parts of England ‘left behind’ by the NHS?” Nuffield Trust comment. Nuffield Trust. 2018.https://www.nuffieldtrust.org.uk/news-item/are-parts-of-england-left-behind-by-the-nhs (accessed Sep 2021).
- 8Todd A, Copeland A, Husband A, et al. The positive pharmacy care law: an area-level analysis of the relationship between community pharmacy distribution, urbanity and social deprivation in England. BMJ Open 2014;4:e005764–e005764. doi:10.1136/bmjopen-2014-005764
- 9Ayorinde AA, Porteous T, Sharma P. Screening for major diseases in community pharmacies: a systematic review†. International Journal of Pharmacy Practice 2013;21:349–61. doi:10.1111/ijpp.12041
- 10Perraudin C, Bugnon O, Pelletier-Fleury N. Expanding professional pharmacy services in European community setting: Is it cost-effective? A systematic review for health policy considerations. Health Policy 2016;120:1350–62. doi:10.1016/j.healthpol.2016.09.013
- 11Lindsey L, Husband A, Nazar H, et al. Promoting the early detection of cancer: A systematic review of community pharmacy-based education and screening interventions. Cancer Epidemiology 2015;39:673–81. doi:10.1016/j.canep.2015.07.011
- 12Willis A, Rivers P, Gray LJ, et al. The Effectiveness of Screening for Diabetes and Cardiovascular Disease Risk Factors in a Community Pharmacy Setting. PLoS ONE 2014;9:e91157. doi:10.1371/journal.pone.0091157
- 13Buchanan R, Cooper K, Grellier L, et al. The testing of people with any risk factor for hepatitis C in community pharmacies is cost‐effective. J Viral Hepat 2019;27:36–44. doi:10.1111/jvh.13207
- 14Integrating community pharmacy testing for hepatitis C with specialist care. Pharmaceutical Journal Published Online First: 2016. doi:10.1211/pj.2016.20201549
- 15Diagnosing viral hepatitis in the community A 3-month pharmacy testing pilot. The Hepatitis C Trust. 2010.http://www.hcvaction.org.uk/resource/diagnosing-viral-hepatitis-community-3-month-pharmacy-testing-pilot (accessed Sep 2021).
- 16Radley A, de Bruin M, Inglis SK, et al. Clinical effectiveness of pharmacist-led versus conventionally delivered antiviral treatment for hepatitis C virus in patients receiving opioid substitution therapy: a pragmatic, cluster-randomised trial. The Lancet Gastroenterology & Hepatology 2020;5:809–18. doi:10.1016/s2468-1253(20)30120-5
- 17Radley A, Tait J, Dillon JF. DOT-C: A cluster randomised feasibility trial evaluating d irectly o bserved an t i-H C V therapy in a population receiving opioid substitute therapy from community pharmacy. International Journal of Drug Policy 2017;47:126–36. doi:10.1016/j.drugpo.2017.05.042
- 18Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. The Lancet 2014;384:1953–97. doi:10.1016/s0140-6736(14)61838-9
- 19Living Well for Longer: A call to action to reduce avoidable premature mortality . Department of Health. 2013.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/181103/Living_well_for_longer.pdf (accessed Sep 2021).
- 20Williams R, Ashton K, Aspinall R, et al. Implementation of the Lancet Standing Commission on Liver Disease in the UK. The Lancet 2015;386:2098–111. doi:10.1016/s0140-6736(15)00680-7
- 21Williams R, Alexander G, Aspinall R, et al. New metrics for the Lancet Standing Commission on Liver Disease in the UK. The Lancet 2017;389:2053–80. doi:10.1016/s0140-6736(16)32234-6
- 22Research Priorities for Alcohol-Related Liver Disease. The James Lind Alliance. 2016.https://www.jla.nihr.ac.uk/priority-setting-partnerships/alcohol-related-liver-disease/ (accessed Sep 2021).
- 23Number of deaths caused by alcoholic liver disease and other causes associated with the misuse of alcohol, deaths registered in England and Wales, 2016. Office for National Statistics. 2017.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/007370numberofdeathscausedbyalcoholicliverdiseaseandothercausesassociatedwiththemisuseofalcoholdeathsregisteredinenglandandwales2016 (accessed Sep 2021).
- 24Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 2015;386:2287–323. doi:10.1016/s0140-6736(15)00128-2
- 25Working years of life lost due to alcohol mortality. Public Health England. 2020.https://www.gov.uk/government/publications/working-years-of-life-lost-due-to-alcohol-mortality (accessed Sep 2021).
- 26Cargill Z, Kattiparambil S, Hansi N, et al. Severe alcohol-related liver disease admissions post-COVID-19 lockdown: canary in the coal mine? Frontline Gastroenterol 2020;12:354–5. doi:10.1136/flgastro-2020-101693
- 27How to provide advice on alcohol consumption and explain the potential health risks. Pharmaceutical Journal Published Online First: 2020. doi:10.1211/pj.2020.20207992
- 28Services Database. Pharmaceutical Services Negotiating Committee. https://psnc.org.uk/services-commissioning/services-database (accessed Sep 2021).
- 29WHO alcohol brief intervention training manual for primary care. World Health Organization. 2017.https://www.euro.who.int/en/health-topics/disease-prevention/alcohol-use/publications/2017/who-alcohol-brief-intervention-training-manual-for-primary-care-2017 (accessed Sep 2021).
- 30UK Chief Medical Officers’ Low Risk Drinking Guidelines . Department of Health . 2016.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/545937/UK_CMOs__report.pdf (accessed Sep 2021).
- 31Alcohol units. NHS. 2018.https://www.nhs.uk/live-well/alcohol-support/calculating-alcohol-units (accessed Sep 2021).
- 32Alcohol-use disorders: prevention. Public Health Guideline [PH24] . National Institute for Health and Care Excellence. 2010.https://www.nice.org.uk/guidance/ph24 (accessed Sep 2021).
- 33The international statistical classification of diseases and related health problems, ICD-10. World Health Organization. 2018.https://icd.who.int/browse10/2019/en (accessed Sep 2021).
- 34American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2013. doi:10.1176/appi.books.9780890425596
- 35SAUNDERS JB, AASLAND OG, BABOR TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993;88:791–804. doi:10.1111/j.1360-0443.1993.tb02093.x
- 36Thursz M, Gual A, Lackner C, et al. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. Journal of Hepatology 2018;69:154–81. doi:10.1016/j.jhep.2018.03.018
- 37Alcohol use screening tests. Public Health England. 2017.https://www.gov.uk/government/publications/alcohol-use-screening-tests (accessed Sep 2021).
- 38Guidance on the 5 alcohol use screening tests . Public Health England. 2017.https://www.gov.uk/government/publications/alcohol-use-screening-tests/guidance-on-the-5-alcohol-use-screening-tests (accessed Sep 2021).
- 39Avila MA, Dufour J-F, Gerbes AL, et al. Recent advances in alcohol-related liver disease (ALD): summary of a Gut round table meeting. Gut 2019;69:764–80. doi:10.1136/gutjnl-2019-319720
- 40Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018;4. doi:10.1038/s41572-018-0014-7
- 41Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut 2017;67:6–19. doi:10.1136/gutjnl-2017-314924
- 42Ratib S, Fleming KM, Crooks CJ, et al. 1 and 5 year survival estimates for people with cirrhosis of the liver in England, 1998–2009: A large population study. Journal of Hepatology 2014;60:282–9. doi:10.1016/j.jhep.2013.09.027
- 43Sheron N, Moore M, O’Brien W, et al. Feasibility of detection and intervention for alcohol-related liver disease in the community: the Alcohol and Liver Disease Detection study (ALDDeS). Br J Gen Pract 2013;63:e698–705. doi:10.3399/bjgp13x673711
- 44Teli MR, Day CP, James OFW, et al. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. The Lancet 1995;346:987–90. doi:10.1016/s0140-6736(95)91685-7
- 45Verrill C, Markham H, Templeton A, et al. Alcohol-related cirrhosis-early abstinence is a key factor in prognosis, even in the most severe cases. Addiction 2009;104:768–74. doi:10.1111/j.1360-0443.2009.02521.x
- 46Hazeldine S, Hydes T, Sheron N. Alcoholic liver disease – the extent of the problem and what you can do about it. Clin Med 2015;15:179–85. doi:10.7861/clinmedicine.15-2-179
- 47Alcohol-use disorders: diagnosis, assessment and management of harmful drinking (high-risk drinking) and alcohol dependence. Clinical guideline [CG115]. . National Institute for Health and Care Excellence. 2011.www.nice.org.uk/guidance/cg115 (accessed Sep 2021).
- 48Kaner EF, Beyer FR, Muirhead C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database of Systematic Reviews 2018;2018. doi:10.1002/14651858.cd004148.pub4
- 49Hadefi A, Degré D, Trépo E, et al. Noninvasive diagnosis in alcohol‐related liver disease. Health Sci Rep 2020;3. doi:10.1002/hsr2.146
- 50EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. Journal of Hepatology 2015;63:237–64. doi:10.1016/j.jhep.2015.04.006
- 51Cirrhosis in over 16s: assessment and management. NICE Guideline [NG50]. National Institute for Health and Care Excellence. 2016.https://www.nice.org.uk/guidance/ng50 (accessed Sep 2021).
- 52FibroScan for assessing liver fibrosis and cirrhosis in primary. Medtech innovation briefing [MIB216]. National Institute for Health and Care Excellence. 2020.www.nice.org.uk/guidance/mib216 (accessed Sep 2021).
- 53NHS Health Check Best practice guidance. Public Health England. 2019.https://www.healthcheck.nhs.uk/seecmsfile/?id=1480 (accessed Sep 2021).
- 54Chalmers J, Wilkes E, Harris R, et al. Development and implementation of a commissioned pathway for the identification and stratification of liver disease in the community. Frontline Gastroenterol 2019;11:86–92. doi:10.1136/flgastro-2019-101177
- 55Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. Journal of Hepatology 2019;71:371–8. doi:10.1016/j.jhep.2019.03.033
- 56Matthews M. Southampton Primary Care Liver Pathway . St Mary’s Surgery. 2021.https://www.stmaryssurgery.nhs.uk/southampton-primary-care-liver-pathway (accessed Sep 2021).
- 57Macpherson I, Nobes JH, Dow E, et al. Intelligent Liver Function Testing: Working Smarter to Improve Patient Outcomes in Liver Disease. The Journal of Applied Laboratory Medicine 2020;5:1090–100. doi:10.1093/jalm/jfaa109
- 58Asphaug L, Thiele M, Krag A, et al. Cost‐Effectiveness of Noninvasive Screening for Alcohol‐Related Liver Fibrosis. Hepatology 2020;71:2093–104. doi:10.1002/hep.30979
- 59Mansfield K, Crellin E, Denholm R, et al. Completeness and validity of alcohol recording in general practice within the UK: a cross-sectional study. BMJ Open 2019;9:e031537. doi:10.1136/bmjopen-2019-031537
- 60Harman DJ, Ryder SD, James MW, et al. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: a cross-sectional diagnostic study utilising transient elastography. BMJ Open 2015;5:e007516–e007516. doi:10.1136/bmjopen-2014-007516
- 61NHS Digital Population Health Team. Health Survey for England 2018: Adult heath related behaviours. NHS Digital. 2019.https://files.digital.nhs.uk/D4/93337C/HSE19-Adult-health-behaviours-rep.pdf (accessed Sep 2021).
- 62Ratib S, West J, Crooks CJ, et al. Diagnosis of Liver Cirrhosis in England, a Cohort Study, 1998–2009: A Comparison With Cancer. American Journal of Gastroenterology 2014;109:190–8. doi:10.1038/ajg.2013.405
- 63Watson M, Inch J, Jaffray M. Screening and brief interventions for alcohol misuse delivered in the community pharmacy setting: a pilot study. Abstract 50. International Journal of Pharmacy Practice 2011;19:4–6. doi:10.1111/j.2042-7174.2011.00098.x
- 64Dhital R, Norman I, Whittlesea C, et al. The effectiveness of brief alcohol interventions delivered by community pharmacists: randomized controlled trial. Addiction 2015;110:1586–94. doi:10.1111/add.12994
- 65Goodall T, Dawson P. A Feasibility Study: The role of Community Pharmacists in the Identification and Treatment of Hazardous Drinking. Leeds PCT. 2006.https://www.alcoholpolicy.net/files/PharmHazDrinkRepJuly7.doc (accessed Sep 2021).
- 66Fitzgerald N, McCaig DJ, Watson H, et al. Development, implementation and evaluation of a pilot project to deliver interventions on alcohol issues in community pharmacies. International Journal of Pharmacy Practice 2008;16:17–22. doi:10.1211/ijpp.16.1.0004
- 67DHITAL R, WHITTLESEA CM, NORMAN IJ, et al. Community pharmacy service users’ views and perceptions of alcohol screening and brief intervention. Drug and Alcohol Review 2010;29:596–602. doi:10.1111/j.1465-3362.2010.00234.x
- 68Sheridan J, Stewart J, Smart R, et al. Risky drinking among community pharmacy customers in New Zealand and their attitudes towards pharmacist screening and brief interventions. Drug Alcohol Rev 2012;31:56–63. doi:10.1111/j.1465-3362.2011.00293.x
- 69Khan NS, Norman IJ, Dhital R, et al. Alcohol brief intervention in community pharmacies: a feasibility study of outcomes and customer experiences. Int J Clin Pharm 2013;35:1178–87. doi:10.1007/s11096-013-9845-1
- 70Brown S, Henderson E, Sullivan C. The feasibility and acceptability of the provision of alcohol screening and brief advice in pharmacies for women accessing emergency contraception: an evaluation study. BMC Public Health 2014;14. doi:10.1186/1471-2458-14-1139
- 71Krska J, Mackridge AJ. Involving the public and other stakeholders in development and evaluation of a community pharmacy alcohol screening and brief advice service. Public Health 2014;128:309–16. doi:10.1016/j.puhe.2013.11.001
- 72Hattingh HL, Hallett J, Tait RJ. ‘Making the invisible visible’ through alcohol screening and brief intervention in community pharmacies: an Australian feasibility study. BMC Public Health 2016;16. doi:10.1186/s12889-016-3805-3
- 73Dhital R, Greene R, Lovejoy A. Feasibility of an alcohol screening service in a community pharmacy. International Journal of Pharmacy Practice 2005;13:R84.
- 74Stewart D, van Dongen A, Watson M, et al. A pilot cluster randomised trial of the Medicinesand Alcohol Consultation (MAC): an intervention to discuss alcohol use in community pharmacy medicine review services. BMC Health Serv Res 2020;20. doi:10.1186/s12913-020-05797-z
- 75Service Specification: Community pharmacy Hepatitis C Antibody Testing Service-Advanced Service. NHS England and NHS Improvement/PSNC. 2020.https://www.england.nhs.uk/publication/service-specification-community-pharmacy-hepatitis-c-antibody-testing-service-advanced-service (accessed Sep 2021).
- 76Buchanan R, Sinclair JMA. Alcohol use disorder and the liver. Addiction 2020;116:1270–8. doi:10.1111/add.15204
- 77Williams R, Alexander G, Aspinall R, et al. Gathering momentum for the way ahead: fifth report of the Lancet Standing Commission on Liver Disease in the UK. The Lancet 2018;392:2398–412. doi:10.1016/s0140-6736(18)32561-3