Shutterstock.com
After reading this article, you should be able to:
- Understand how prematurity is classified by gestational age and weight;
- Appreciate the effect of prematurity on organ systems;
- Understand how to manage common neonatal issues.
A full-term pregnancy is defined as a baby born between 37 and 42 weeks gestational age1.
The World Health Organization classifies any baby born before 37 weeks as a preterm baby2: babies born between 27 to 32 weeks are classified as very preterm; and babies born between 22 to 26 weeks are classified as extremely preterm2.
Complications associated with prematurity are usually more apparent in babies born extremely prematurely. The British Association of Perinatal Medicine (BAPM) 2019 framework — ‘Perinatal Management of Extreme Preterm Birth’ — was developed to help guide healthcare professionals on how best to manage the care of these babies and their families3. The framework includes a helpful infographic, seen in Figure 1, which outlines the expected outcomes in terms of morbidity and mortality for babies born at this stage of their development3:
When talking about babies who have been born extremely prematurely, the term ‘severe disability’ includes factors such as:
- Not being able to walk or get around independently (including conditions such as severe cerebral palsy);
- Being unable to talk, or see or hear properly;
- Difficulties with swallowing or feeding safely;
- Having multiple health problems with frequent visits to hospital;
- Needing to attend a separate school for children with special educational needs;
- Being unable to care for themselves or live independently as they grow up3.
Complications in infants born after 26 weeks gestational age can still be significant – they are not limited to babies born under 26 weeks.
One of the most apparent features of a baby born prematurely is their reduced size and weight. In the UK, a baby born at term has an average weight of 3.5kg, whereas the smallest baby to have been born prematurely and survived weighed just 305g4.
Neonatal weight is usually categorised as follows:
- Low birth weight (<2,500g);
- Very low birth weight (<1,500g);
- Extremely low birth weight (<1,000g)5.
Owing to the fact that premature babies have immature organs and underdeveloped systems (e.g. immune function), they are at higher risk of complications6. Common problems are observed concerning respiratory function, liver function, infection, cardiovascular, the central nervous and endocrine systems. Information about complications relating to gastrointestinal function can be found in ‘Gastrointestinal complications associated with prematurity‘.
Additional detail on types of neonatal units and pharmacists role in caring for neonates can be found in ‘Best practice for medicines use in neonates’
This article will cover how prematurity impacts different organ systems, common neonatal conditions that occur as a result of this, and the pharmaceutical management of these conditions.
Skin care
The skin barrier function develops over the first few weeks of life, especially in very preterm infants (<30 weeks gestation)6,7. In extremely premature babies, it is easier to see a baby’s veins under the skin because they have so little subcutaneous fat and muscle. Preterm infants are at risk of high body water losses owing to trans-epidermal water loss through immature skin — this is why they are cared for in incubators, which help to maintain a high humidity environment8. This means that premature neonates are more prone to skin damage, water loss and infection9. Care must be taken when applying and removing dressings because the skin is very fragile and can damage easily. If adhesives are necessary, hydrogel dressings or silicone-based tapes should be used to minimise trauma10.
When caring for the skin of premature babies, several additional considerations are needed, particularly regarding nappy care and bathing, owing to their delicate and underdeveloped skin. These are summarised in Box 1.
Box 1: Best practice for skincare in premature infants
Nappy care considerations:
- Frequent changing of nappies is necessary. Since premature babies have thin skin, prolonged exposure to urine and stool (which contain irritants like proteolytic enzymes and ammonia) can lead to dermatitis11;
- Barrier creams such as zinc oxide or petrolatum-based creams should be used to protect against moisture and irritation12;
- Alcohol-based or fragranced wipes can disrupt skin pH. Instead, soft cotton wool with warm water should be used to clean the skin13;
- Premature infants are more susceptible to Candida (yeast) infections, which can present as a red, persistent rash14.
Bathing considerations
- Minimal bathing is recommended in the first weeks. Excessive bathing can cause skin barrier disruption and dehydration;
- Mild, pH-balanced cleansers should be used. Baby skin has a higher pH (around 6.5 at birth, lowering to 5.5 over time). Alkaline soaps should be avoided — these can disrupt the skin microbiome and increase the risk of irritation and infection15;
- Scrubbing should be avoided. The skin should be patted dry instead of rubbing;
- Water should be kept warm to prevent hypothermia, because premature infants have poor temperature regulation16.
Moisturisation
- Regular use of fragrance-free emollients (e.g. medical-grade sunflower seed oil) helps to prevent dry skin and cracking, which can be an entry point for infections;
- Certain plant-based oils (e.g. olive oil) contain oleic acid, which can weaken the skin barrier17.
Preterm neonates are at increased risk of drug absorption owing to the permeability of their skin; therefore, preparations containing iodine should be avoided because they can disrupt thyroid function18. Topical steroids are also usually avoided, owing to increased risk of skin thinning and adrenal dysfunction19. Chlorhexidine, which is a commonly applied agent for neonatal skin decontamination, can potentially cause severe harm20,21.
In 2021, the Neonatal and Paediatric Pharmacy Group (NPPG) issued a position statement regarding the appropriate strength of chlorhexidine solutions for skin cleansing in neonates. This guidance, endorsed by BAPM, provides specific recommendations based on gestational age and post-natal age.
For neonates born before 34 weeks’ gestation and under 7 days old:
- A 0.5% aqueous chlorhexidine gluconate solution should be used for skin preparation. A licensed 0.5% aqueous chlorhexidine solution is not currently available in the UK. The NPPG has expressed the need for pharmaceutical manufacturers to develop licensed 0.5% aqueous chlorhexidine products to ensure consistency and safety in neonatal care.
For neonates born from 34 weeks’ gestation onwards, and for those born before 34 weeks’ gestation who are now 7 days or older:
- A 2% chlorhexidine solution in isopropyl alcohol should be used for skin preparation22.
Regardless of the solution used, it is crucial to prevent pooling on the skin and surfaces that may come into contact with the baby’s skin, such as incubator or cot sheets. To minimise this risk, the use of an applicator device is recommended whenever possible22.
Respiratory care and conditions
In utero, the bronchial tree can usually be visualised by week 16 (see Figure 2).
Between weeks 17 to 26, bronchioles branch further and become more vascularised. At around week 24, surfactant production starts and reaches sufficient amounts by week 3223.
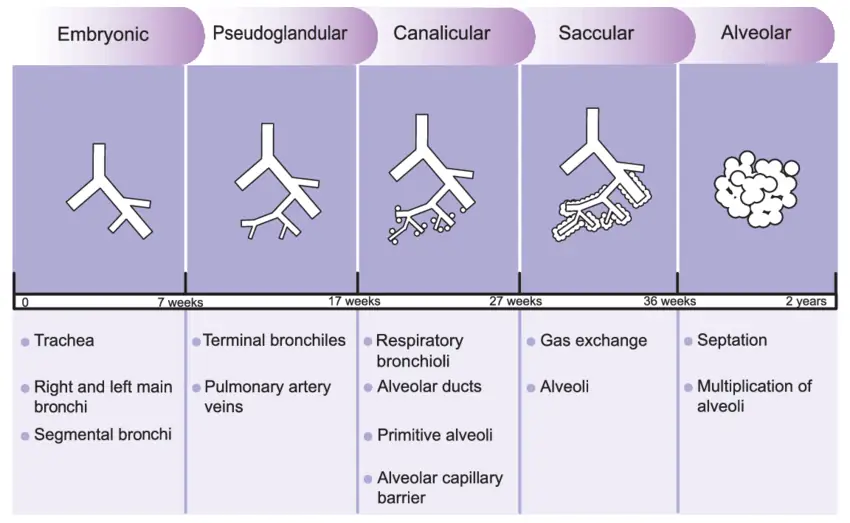
Reproduced with permission from The Journal of Maternal-Fetal & Neonatal Medicine
When a pregnant woman is identified at being at risk of delivering prematurely, a course of IM corticosteroids is administered (typically as two injections), to help the baby’s lungs to mature and for surfactant production to accelerate24.
Antenatal risk factors for preterm delivery (birth before 37 weeks gestation) can be grouped into several categories. Examples include, but are not limited to: maternal factors (e.g. history of preterm birth); obstetric and gynaecological factors (e.g. history of recurrent miscarriages); and infections and medical conditions (e.g. maternal infections, such as bacterial vaginosis, urinary tract infections or sexually transmitted infections)25.
A 2020 Cochrane review demonstrated that giving steroids reduces the risks of peri-and neonatal deaths, and breathing problems in the first few hours of life26.
Use of pulmonary surfactants
Pulmonary surfactant is a complex mixture of lipids and proteins necessary for alveolar stability and gas exchange27. Premature neonates born before 32 weeks, are at higher risk of developing respiratory distress, owing to a lack of endogenous surfactant28–30. The underdeveloped lungs with their immature microstructure, and inflammation caused by exposure to damaging exogenous influences, such as oxygen and pressure from ventilators, are also contributing factors to respiratory distress31.
Clinical indications for administration of surfactant include:
- Neonates with clinical or radiological evidence of respiratory distress syndrome (RDS);
- Neonates at risk of developing RDS (e.g. under 32 weeks corrected gestational age);
- Intubated neonates whose fraction of inspired oxygen (FiO2 level) exceeds 0.532.
In the UK, the National Institute for Health and Care Excellence (NICE) provides dosing guidelines for poractant alfa (Curosurf; Chiesi) in the British National Formulary for Children (BNFC). Poractant alfa lowers the surface tension at the air-liquid interface within the alveoli and prevents their collapse during exhalation. It mimics the action of endogenous surfactant and restores lung function. For neonates with a birth weight above 700g, the recommended initial dose is 100–200mg/kg, administered via endotracheal tube within 15 minutes of birth. If required, additional doses of 100 mg/kg may be given every 12 hours, with a maximum total dose of 300–400mg/kg per course33.
Less invasive surfactant administration (LISA) has been developed in recent years as an alternative method of administering pulmonary surfactant. Rather than insertion via an endotracheal tube, this technique uses a thin catheter to deliver surfactant in a spontaneously breathing infant on continuous positive airway pressure (CPAP). Where adequate expertise is available, LISA should be the preferred method of surfactant administration, as it is associated with improved outcomes34.
Respiratory support
In 2019, NICE published a quality standard on ‘Specialist neonatal respiratory care for babies born preterm’ and outlined five key quality statements on respiratory support soon after birth; less invasive surfactant administration; volume-targeted and synchronised ventilation; target oxygen saturation; and parental involvement in care35. Please consult the quality standard for more information.
The NICE standard recommends that babies that require respiratory support should be given CPAP, where clinically appropriate, rather than invasive ventilation. This helps to lower the risk of the baby developing bronchopulmonary dysplasia (BPD). CPAP machines and ventilators both provide respiratory support, but they differ in function, application and the level of assistance they provide (see Table36). In neonatal care, CPAP is often the first-line intervention to support breathing, while ventilators are used when CPAP is insufficient.
Neonatal apnoea
Neonatal apnoea is defined as an unexplained episode of cessation of breathing and is usually identified by apnoea alarms that are placed inside the cot. It is commonly observed in preterm neonates owing to poor development of the mechanisms involved in respiratory control37.
The incidence varies depending on gestational age:
- Nearly all extremely preterm infants (<28 weeks gestation) experience some degree of apnoea;
- About 50–80% of very preterm infants (28–32 weeks gestation) are affected;
- Less common in moderate-to-late preterm infants (32–36 weeks gestation), but still occurs in 10–50% of cases;
- Rare in term infants (>37 weeks gestation), usually linked to underlying conditions such as infection, metabolic disorders or neurological issues38.
Neonatal apnoea usually resolves by 36/37 weeks gestational age as the infant’s respiratory control matures38.
Physical stimulation often helps to resolve apnoea36. To minimise the risk of apnoea developing, CPAP is given and caffeine citrate is administered routinely to all babies under 28 weeks gestational age39. Typically, a loading dose of 20mg/kg, followed by a maintenance dose of 5–10mg/kg/day, adjusted as needed40. For babies of 28–32 weeks gestation, caffeine citrate is still commonly used, but is based on clinical assessment rather than automatic administration41.
Pharmacists must ensure that the dose is expressed in terms of caffeine citrate rather than caffeine base because the two forms have different strengths39. Most neonatal guidelines, including those from BNFC and neonatal networks, recommend dosing in caffeine citrate to avoid confusion. Caffeine citrate is approximately twice the weight of caffeine base; therefore, misinterpretation — such as prescribing 2mg/kg as caffeine base instead of caffeine citrate — could lead to a two-fold overdose.
Caffeine citrate is usually given intravenously for the first few days of life, then switched to the enteral route once enteral feeding has been established.
Bronchopulmonary dysplasia
One of the long-term respiratory complications associated with prematurity is bronchopulmonary dysplasia (BPD)42, which is defined as the requirement of respiratory support at 36 weeks post-menstrual age in an infant born before 32 weeks43.
BPD is a significant complication among preterm infants in the UK — its incidence is closely linked to gestational age and birth weight:
- Around 45% of infants born before 28 weeks’ gestation develop BPD;
- Around 37% of infants born before 32 weeks’ gestation are affected;
- Infants with birth weights under 1250g account for 97% of all BPD cases44.
Despite advancements in neonatal care, the incidence of BPD has remained static or has even increased, underscoring the need for continued research and improved management strategies45. Efforts are ongoing to identify and implement best practices aimed at reducing the incidence of BPD in preterm infants.
BPD has a significant impact on mortality and long-term morbidities, and associated healthcare costs, and it is still the most frequent long-term adverse outcome for babies born prematurely, despite several interventions being tried to reduce the incidence globally43. In December 2023, BAPM published a quality improvement toolkit on ‘Reducing the incidence of bronchopulmonary dysplasia’, and the pharmacological interventions recommended are:
- Caffeine citrate: should be started within 24 hours (to reduce incidence of neonatal apnoea);
- Low-dose postnatal hydrocortisone for babies born below 28 weeks of gestation (from day 1 of life). There are limited data available for babies under 24 weeks and the use of hydrocortisone should be avoided in babies on ibuprofen or indomethacin, owing to increased risks of renal and gastrointestinal complications43,46.
Other interventions that may be considered to help lower the risk of BPD include postnatal administration of steroids, such as dexamethasone (in preterm infants who are 8 days or older, and still need invasive ventilation)47–49.
The DART protocol (‘Dexamethasone: a randomised trial’) refers to a regimen of dexamethasone therapy typically administered to preterm infants who are at risk of BPD or other complications related to prematurity50. The protocol typically involves 0.89mg/kg of dexamethasone given over 10 days, aimed at reducing inflammation in the lungs to prevent or treat BPD. The risks and benefits need to be discussed with parents.
The DART protocol offers benefits in terms of reducing the incidence of BPD and improving respiratory outcomes in preterm infants, especially those born very preterm or with a high risk of lung disease; however, the risks associated with corticosteroid use — such as neurodevelopmental delays, growth restriction, and infection — need to be carefully weighed against the potential benefits, and the protocol must be used with caution51,52. Pharmacists must closely monitor infants for any signs of side effects while following the protocol and use dexamethasone for the shortest duration necessary to avoid long-term complications.
No high-quality evidence supports the routine use of diuretics to reduce the incidence of BPD, although short-term improvements may be seen in babies with pulmonary oedema43.
Cardiovascular complications
The hearts of babies born prematurely develop differently following birth and prematurity is associated with an increased incidence of cardiovascular conditions in adulthood53.
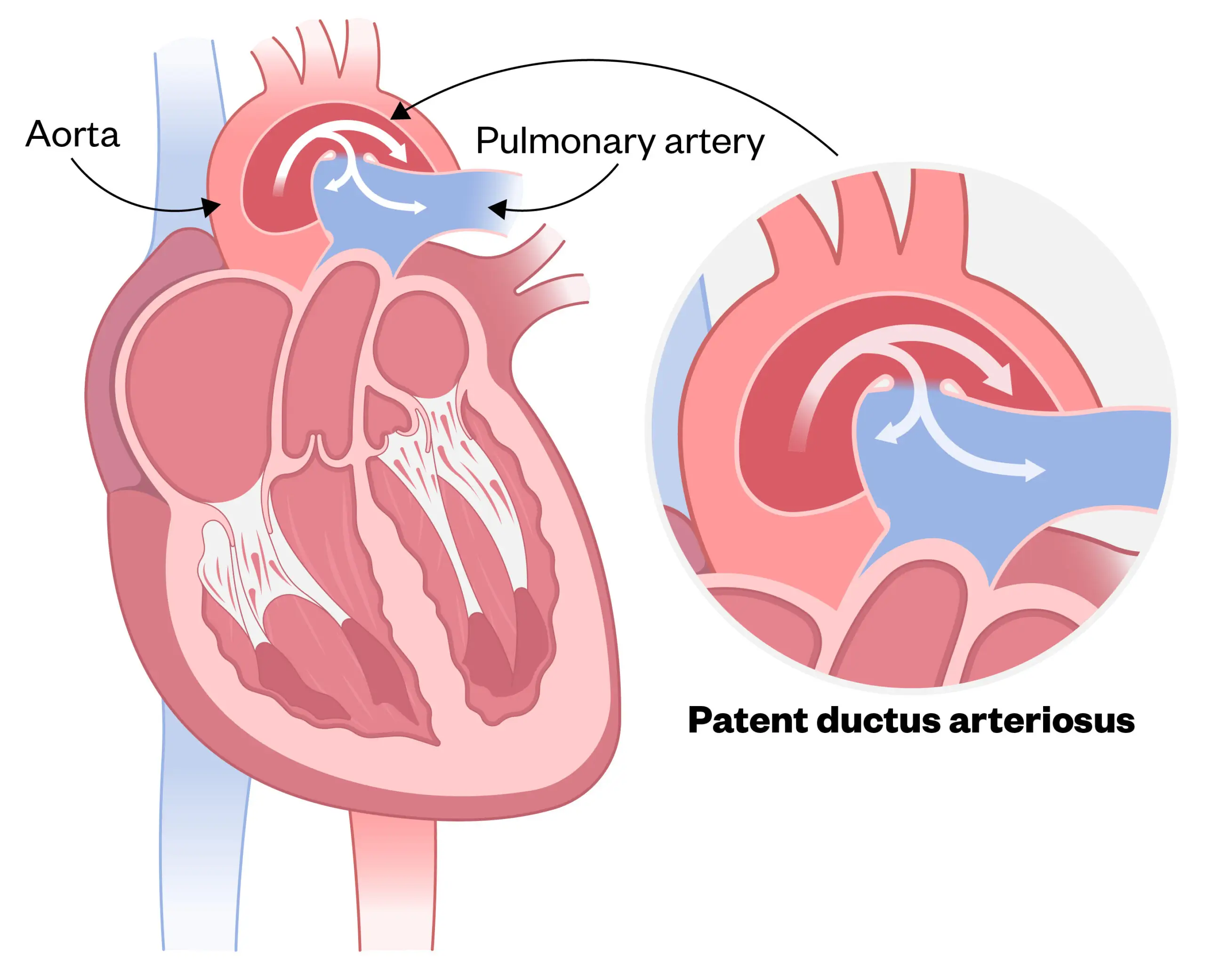
Patent ductus arteriosus
Patent ductus arteriosus (PDA) is the most common cardiovascular complication seen in preterm neonates. The reported incidence of PDA in term neonates is only 1 in 2,000 births, accounting for 5–10% of all congenital heart disease. The incidence of PDA in preterm neonates is far greater, with reports ranging from 20%–60%. PDA is present in 80% of infants weighing less than 1,200g at birth, compared with 40% of infants weighing less than 2,000g at birth54.
Results of a 2022 retrospective cohort study involving 58,108 infants born <32 weeks’ gestational age in England and Wales between 2010 and 2017 showed that 28.3% infants had a PDA diagnosis, either clinically or with echocardiographic confirmation55.
PDA occurs when there is a persistent opening between the aorta and pulmonary artery, as outlined in Figure 356.
Shutterstock.com
In most term infants, during the first three days of life, this duct closes following a change in the systemic blood flow, a gradual decrease in pulmonary pressure, an increase in arterial oxygen tension and a decreased level of circulating prostaglandins57. However, in preterm infants, this duct re-modelling frequently does not happen owing to the immaturity of the closure systems58.
The main complication associated with a PDA is that a higher amount of blood travels to the lungs, increasing the risks of pulmonary hypertension and right-sided heart failure. PDA can also lead to deoxygenated blood travelling around the body, leading to decreased oxygen perfusion in areas such as the gastrointestinal tract, increasing the risk of NEC59.
Neonatologists may suspect that a neonate has a PDA if they are having difficulties coming off a ventilator or a CPAP machine. The most common sign of a PDA is a heart murmur, which can be detected by a stethoscope and confirmed by an echocardiogram60. A baby with a small PDA may have no signs at all, and in most cases does not require treatment, because it often closes on its own without causing significant problems.
Unless a small PDA is causing problems, watchful waiting is usually the best approach rather than immediate medical or surgical intervention. However, a baby with a large PDA may experience the following symptoms:
- Tachycardia;
- Hypotension;
- Hypoxemia;
- Apnoea;
- Poor feeding or poor weight gain54.
Since increased levels of circulating prostaglandins have been identified as one of the contributing factors in maintaining the patency of PDAs, prostaglandin inhibitors — such as non-steroidal anti-inflammatory drugs (NSAIDs) — are used to attempt to close the duct medically54. In the UK, ibuprofen is the preferred prostaglandin inhibitor, as its adverse effect profile is more favourable (e.g. lower rates of renal insufficiency, lower incidence of NEC) when compared with indomethacin, and there is a licensed intravenous ibuprofen preparation available61. Results from a 2018 meta-analysis suggest that oral ibuprofen may be more effective than IV62; however there are no licensed oral formulations of ibuprofen for PDA management in the UK, so any use of oral ibuprofen to treat PDAs is considered ‘off-label’.
Some clinicians prescribe medication to reduce gastric acid secretion, such as proton pump inhibitors (e.g. omeprazole) as gastric protection during NSAID treatment for PDA; however, there is evidence that reducing gastric acid in preterm infants may be harmful and has been associated with increased risk of sepsis and NEC63. Giving a small amount of milk (trophic feeds) may be a better way to provide gastric protection.
A 2022 Cochrane review compared the effectiveness of paracetamol in treating PDAs against indomethacin and ibuprofen, and found that the outcomes were similar, with fewer associated adverse effects64. This has led to some centres adopting paracetamol as their first-line treatment for PDA management, but others use it as a second-line agent where NSAIDs are contraindicated. There is currently no licensed formulation of paracetamol available in the UK for this indication, and there is some debate over the dose and monitoring requirements (e.g. whether blood paracetamol levels should be measured)65. If medical treatment fails, the infant may be referred to a cardiac centre for a surgical ligation of the duct66,67.
Hypotension
In extremely preterm infants (<28 weeks gestation), hypotension is very common, affecting 15–50% of neonates in the first few days of life68. In late preterm and term infants (>32 weeks gestation), hypotension is much less common, unless associated with conditions like sepsis, birth asphyxia or congenital heart disease. Causes of hypotension in neonates include:
- Immature cardiovascular system;
- Sepsis or infection;
- Birth asphyxia;
- Patent ductus arteriosus (PDA);
- Adrenal insufficiency;
- Blood loss (e.g., placental abruption, twin-to-twin transfusion syndrome)69.
In mild cases of hypotension, babies can often be monitored without intervention, as blood pressure may stabilise naturally. Moderate to severe cases may require fluid resuscitation, inotropes (dopamine, dobutamine), corticosteroids or blood transfusion, depending on the cause70.
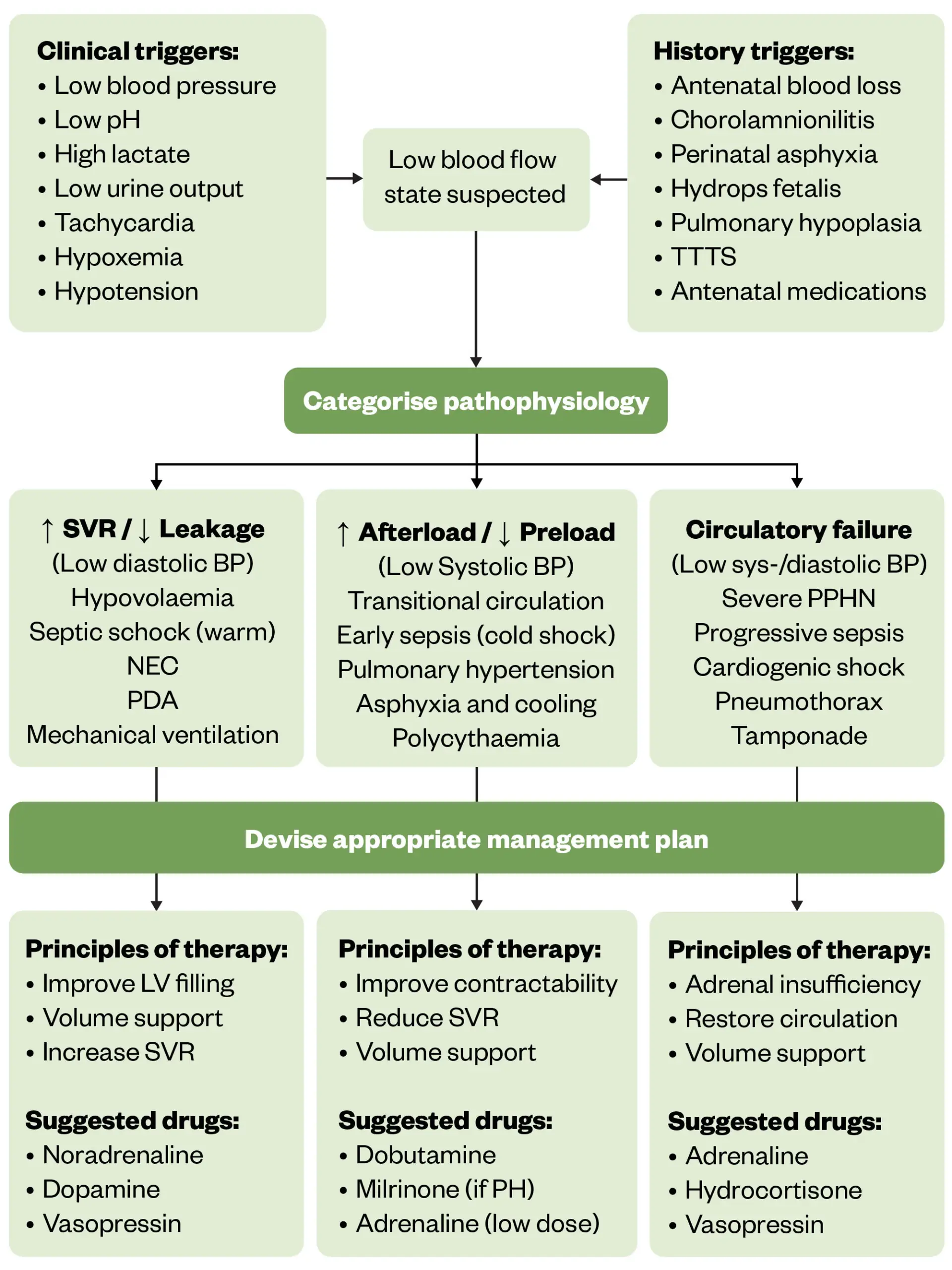
Aggressive treatment of hypotension may be required to preserve organ function; left untreated, hypotension can lead to ventricular haemorrhage, NEC, poor neurological outcomes and mortality71. A bolus dose of isotonic fluid (e.g. sodium chloride 0.9%) is usually administered first, to correct hypovolaemia. If ineffective, an individualised approach should then follow, this will involve selecting the most appropriate inotrope to use depending on what the probable cause of hypotension is. Figure 4 below outlines a suggested approach to managing hypotension in preterm infants72. Treatment options include dopamine, dobutamine, adrenaline, noradrenaline, milrinone, vasopressin and hydrocortisone:
- Dopamine is commonly used for sepsis, particularly in low doses to enhance perfusion and improve heart rate;
- Noradrenaline is used when more potent vasoconstriction is required, especially in septic shock where blood pressure needs to be supported;
- Dobutamine helps with cardiac output and is often used when the issue is cardiogenic in nature (e.g. where there is evidence of myocardial dysfunction or increased systemic vascular resistance)72.
Therapy is adjusted based on clinical monitoring and individual response.
The Pharmaceutical Journal
Refractory adrenal insufficiency is a well-recognised cause of hypotension in sick neonates, so steroids such as hydrocortisone are commonly recommended to treat the adrenal insufficiency and maintain cardiovascular homeostasis73. A typical dosing regimen is 1mg/kg per dose administered intravenously every 8 hours. In cases where the response is inadequate, the frequency may be increased to every 6 hours, with a maximum dose of up to 2mg/kg per dose, as advised by a neonatologist74. It is important to note that hydrocortisone therapy should be tapered gradually after prolonged use to prevent potential adverse effects.
Liver symptoms
Around 50% of term babies and 80% of preterm babies develop jaundice, which usually appears 2 to 4 days after birth75. Babies have a high number of red blood cells, which are broken down frequently, releasing bilirubin into the baby’s blood and tissues76. Jaundice is caused by bilirubin deposition in the skin. A newborn baby’s liver is often not fully mature enough to remove all of the bilirubin from the baby’s blood, causing the yellowing of the skin and the whites of the eyes77. The condition is exacerbated in prematurity.
The main concern associated with untreated jaundice is kernicterus, this is when bilirubin crosses the blood-brain barrier and deposits in the basal ganglia, which can lead to neurotoxicity and potentially death78.
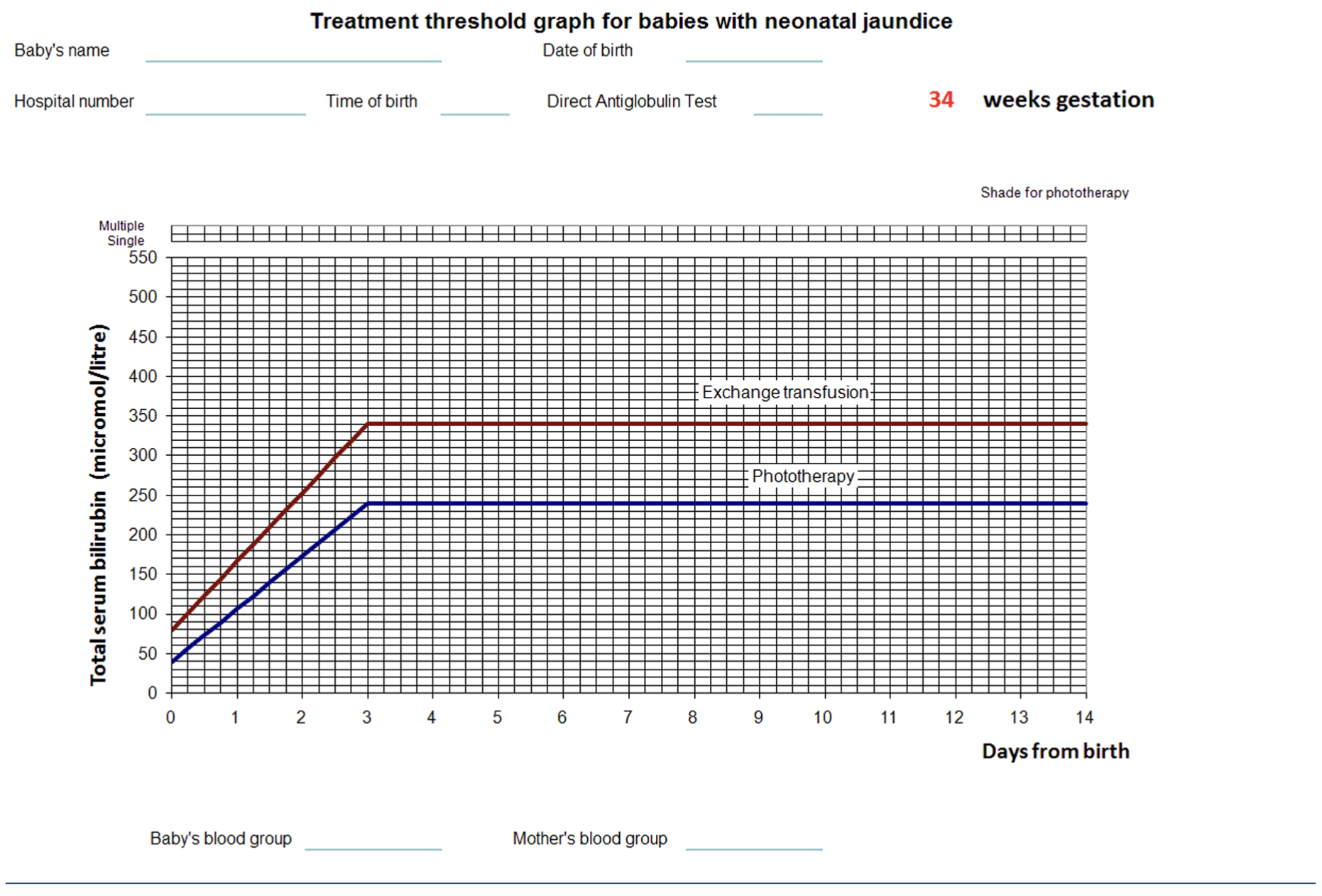
NICE has published guidance on ‘Jaundice in babies under 28 days’, which includes recommendations on how to diagnose and treat jaundice in neonates. Monitoring bilirubin levels is important and values are plotted on treatment threshold graphs, (examples shown in Figure 5 below) to guide treatment79.
Reproduced with permission from National Institute for Health and Care Excellence
Different graphs are recommended for different gestational ages, so it is important to check that the correct graph is being used. Premature infants have a much lower thresholds for treatment than term babies79.
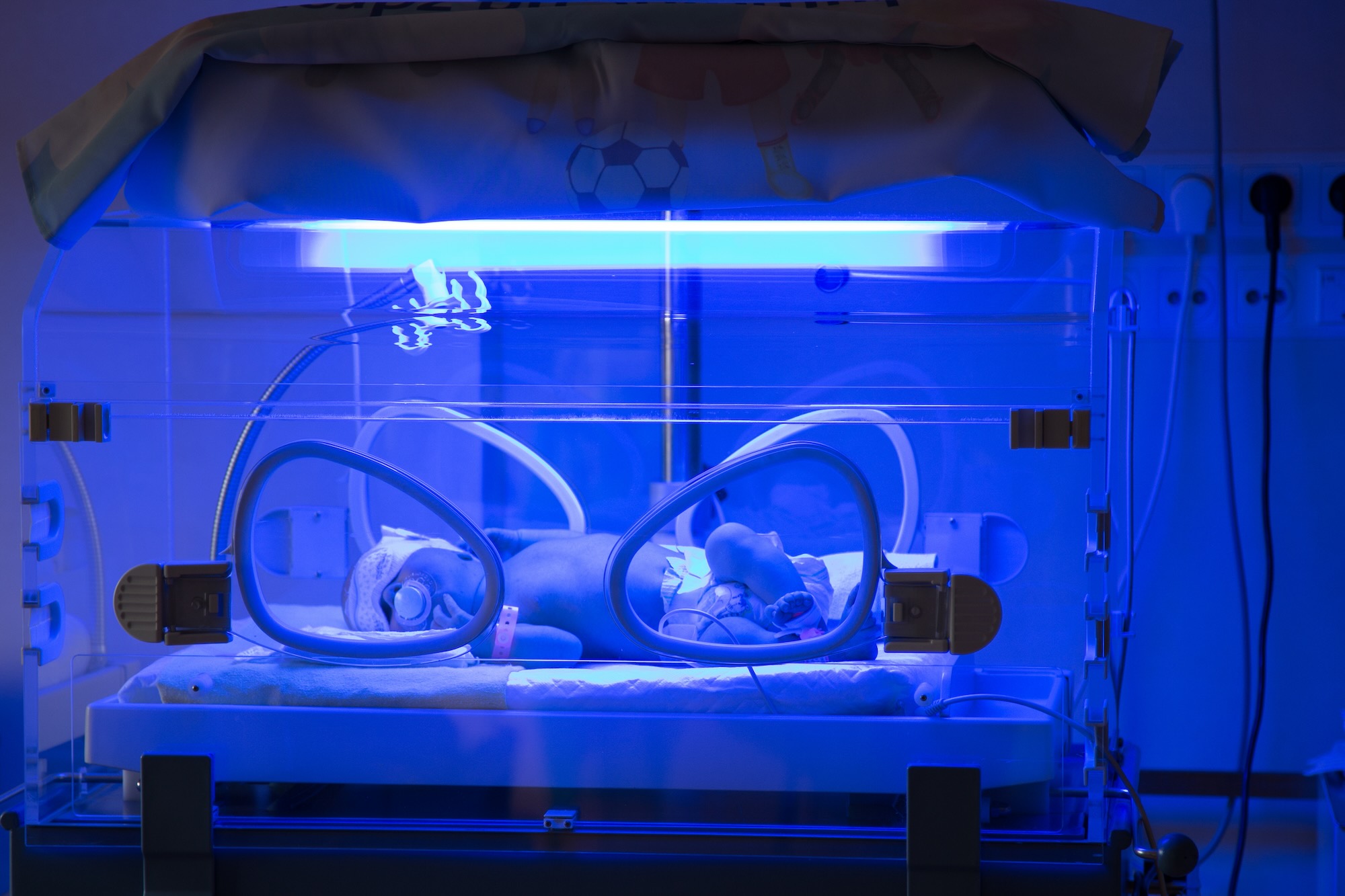
Phototherapy is the first-line option for treating neonatal jaundice, which converts unconjugated bilirubin to a harmless water-soluble pigment by photodegradation through the baby’s skin (see Figure 6)80. Neonatal phototherapy uses blue light in the wavelength range of 430–490nm, with an optimal peak at 460nm. This wavelength is most effective at converting unconjugated bilirubin into water-soluble isomers that can be excreted without requiring liver conjugation.
The duration of phototherapy depends on bilirubin levels, gestational age and response to treatment:
- Mild to moderate hyperbilirubinaemia: Typically, 12–24 hours of phototherapy;
- Severe hyperbilirubinaemia: continuous phototherapy until bilirubin levels drop to a safe range;
- Preterm infants: often require longer treatment than term infants owing to immature liver function81.
Phototherapy is usually stopped when bilirubin levels fall below the treatment threshold and remain stable after 6–12 hours of observation79. Double or triple phototherapy (using multiple light sources) may be used for very high bilirubin levels82. Eye protection is essential to prevent retinal damage79.
Shutterstock.com
Alternative treatments include intravenous immunoglobulins or an exchange transfusion, where the infant’s blood is removed and replaced by donor blood82. IV immunoglobulin reduces the need for exchange transfusion in high-risk infants with haemolytic hyperbilirubinaemia and reduces serum bilirubin levels, the requirement for phototherapy and length of hospital stay83.
Exchange transfusion is considered if bilirubin levels remain dangerously high despite intensive phototherapy. Exchange transfusion has an estimated mortality of 3 to 4 per 1,000 exchanged infants; associated risks include electrolyte and metabolic disturbances (hyperglycaemia, hypocalcaemia, hyponatraemia, hypokalaemia) and thrombocytopenia83.
Eye health
Retinopathy of prematurity (ROP) is a condition affecting the developing blood vessels in the retina of premature infants. The risk increases with lower gestational age and birth weight.
- In extremely preterm infants (<28 weeks gestation), almost all will have some degree of ROP;
- In very preterm infants (28–31 weeks gestation), around 30–50% develop ROP;
- In moderate preterm infants (32–34 weeks gestation), less than 10% develop ROP;
- In late preterm or term infants (>34 weeks gestation), ROP is rare unless other risk factors (e.g. severe illness, high oxygen therapy) are present84.
It is one of the few causes of childhood visual disability and is largely preventable. ROP is caused by disorganised growth of retinal blood vessels, which can result in scarring and retinal detachment85–88. In 2024, the Royal College of Paediatrics and Child Health (RCPCH), Royal College of Ophthalmologists and BAPM updated their joint guideline on screening for retinopathy of prematurity. They recommend screening all babies born under 31 weeks and/or under 1.5kg. Examinations are conducted by trained ophthalmologists; please see the RCPCH ‘Screening of retinopathy of prematurity guideline’ for full list of recommendations and treatment thresholds89.
In the majority of cases, ROP is a mild condition that can spontaneously resolve, but screening is recommended to detect severe disease, to enable any intervention to be made before the child becomes visually impaired90,91.
In preparation for ROP screening, a combination of phenylephrine 2.5% and cyclopentolate 0.5% drops should be administered one hour before, to achieve effective mydriasis89. Prometacaine 0.4% or oxybuprocaine 0.4% drops are recommended for pain relief, if an eye speculum is to be used for the examination89.
In 5–10% of preterm infants, severe ROP is detected and treatment options include laser treatment or off-label intravitreal anti-vascular endothelial growth factor (VEGF) injections, such as bevacizumab92,93. In 2023, NHS England approved ranibizumab for treatment of ROP in the UK, although some neonatal centres are still administering intravitreal bevacizumab94,95. This is because some studies have suggested that recurrence of ROP is higher with ranibizumab than with bevacizumab96.
Neurology
Premature infants are more likely to experience neurological problems because their brains are still in crucial stages of development at the time of birth. The earlier a baby is born, the more vulnerable their brain is to injury and abnormal development. Reasons for this include:
- Incomplete brain development — the brain undergoes rapid growth and maturation during the third trimester. Premature birth interrupts this process, leading to structural and functional immaturity97;
- Underdeveloped blood vessels — the fragile blood vessels in a preterm infant’s brain are more prone to bleeding, which can result in intraventricular haemorrhage (IVH), a serious complication that can damage brain tissue98;
- Lack of myelination — myelin is the protective covering around nerves that helps transmit signals efficiently. Premature babies have less myelination, which can slow neural communication and affect motor and cognitive function99;
- Hypoxia and ischemia — premature infants often struggle with oxygen supply owing to underdeveloped lungs. Low oxygen levels and poor blood flow can cause brain injury, including periventricular leukomalacia (PVL), which affects white matter and increases the risk of cerebral palsy100;
- Increased risk of infections and inflammation — preterm babies are more vulnerable to infections and inflammatory responses, both of which can damage brain tissue and impair neurological development101;
- Nutritional deficiencies — brain development depends on essential nutrients like omega-3 fatty acids, iron and certain vitamins. Premature infants can potentially miss out on these crucial nutrients, which can affect cognitive function102;
- Immature nervous system regulation — premature infants have underdeveloped autonomic nervous system control, making it harder for them to regulate vital functions like breathing and temperature, which can indirectly impact brain health103.
Owing to these factors, preterm infants are at higher risk for cognitive delays, motor impairments (such as cerebral palsy), learning disabilities and behavioural disorders104. However, early medical interventions, therapies and supportive care can improve outcomes105.
Seizures
Neonatal seizures are epileptic fits occurring from birth to the end of the neonatal period. They may be short-lived events, occurring for a few days only. Most (80%) neonatal seizures occur in the first week of life. The incidence is higher in preterm infants compared with term infants106. Babies who experience seizures owing to a brain injury or structural abnormalities may go on to develop cerebral palsy, intellectual disability or other neurological disorders107,108.
Different types of seizure have been observed in the neonatal period, such as subtle, tonic, clonic and myoclonic. Causes of neonatal seizures include:
- Hypoxic ischaemic encephalopathy;
- Intracranial haemorrhage;
- Intracranial infections;
- Congenital cerebral malformations;
- Metabolic disorders (e.g. biotinidase and holocarboxylase synthetase deficiency, cerebral folate deficiency, creatine disorders folinic acid-responsive seizures, glucose transporter type 1 deficiency, mitochondrial disorders, peroxisomal disorders);
- Focal ischaemic stroke106.
In the UK, there are several local network and hospital guidelines for the management of neonatal seizures and in 2022, NHS Scotland published a national guideline. They recommend treating neonates who experience:
- Seizures lasting more than 3 minutes;
- More than 3 seizures in an hour;
- Seizures that result in cardio-respiratory compromise;
- Clinical or electrographic seizures totalling 10 minutes within 60 minutes109.
Very little has changed in the management of neonatal seizures over the past 50 years and the medications used can cause severe side effects, such as sedation, respiratory distress, cardiotoxicity and hepatotoxicity110.
First-line management is a loading dose of phenobarbital over 30 minutes. If this is unsuccessful at terminating the seizure, a second half-load or full-loading dose can be administered109,111.
Second-line management consists of either a levetiracetam or a phenytoin loading dose109,112. If the seizure does not terminate, a midazolam infusion can be started, up to a maximum dose of 300micrograms/kg/hour. As midazolam can cause respiratory depression, the neonate should be electively ventilated, if they are not already109. In preterm neonates, midazolam itself has been associated with abnormal movements, so many clinicians will try to avoid its use in this population113,114.
If a metabolic cause is suspected, pyridoxine can be administered as a trial for 3 days109. Some metabolic teams also recommend giving pyridoxal-5-phosphate, calcium folinate or biotin to see whether the seizures are being triggered by a deficiency115. If all other steps have failed to terminate a seizure, a lidocaine infusion may be considered; however, lidocaine can cause arrythmias, so the neonate must be monitored closely116,117.
Metabolic bone disease
Metabolic bone disease of prematurity (MBDP) is a condition that affects preterm infants and it is characterised by impaired bone mineralisation, owing to inadequate calcium, phosphate and vitamin D intake. It increases the risk of fractures and long-term bone complications118.
Foetal bone mineralisation mainly occurs in the third trimester and babies most at risk of developing MBDP are those119:
- Born under 32 weeks GA and under 1.5kg at birth;
- Who are on prolonged parenteral nutrition (without adequate calcium and phosphate);
- Who have chronic illnesses, such as BPD and NEC;
- Who take medications such as diuretics, steroids and caffeine during pregnancy118,119.
Pharmacists working in neonatal units should:
- Liaise with the neonatal dietician(s) to ensure that calcium, phosphate and vitamin D intake is sufficient and meets ESPGHAN and BDA recommendations120,121. Breast milk can be fortified or specialist preterm formulas can be given (if necessary), which contain extra calcium, phosphate and vitamin D122;
- Minimise the use of medicines that promote calcium loss (e.g. furosemide) and monitor or adjust steroid therapy to prevent bone loss123;
- Monitor serum markers such as alkaline phosphatase and parathyroid hormone levels, which may indicate that MBDP is evolving124.
BAPM are currently developing a quality improvement toolkit on the prevention and management of MBDP and once it is published, this will assist the neonatal multidisciplinary team with applying the best evidence-based approaches to reduce the incidence of MBDP and improve outcomes.
Best practice for pharmacists
- Familiarise yourself with local and national neonatal guidelines, so that you understand how to apply them in a clinical setting;
- Collaboration is crucial — if you are the only one in the pharmacy team managing the care of neonates (e.g. in a district general hospital) reach out for help and support. The local neonatal operational delivery network may employ a lead pharmacist for the area, or there are national organisations you can join, such as the NPPG’s Neonatology Special Interest Group or BAPM;
- Improving services for neonatal patients and their families is vital – look for opportunities to participate in multidisciplinary research, audit (e.g. adherence to local/national recommendations) or quality improvement projects;
- Be aware of the risks associated with transitions of care (e.g. when a baby is transferred from the neonatal unit to another ward/hospital, or when a neonate is ready for discharge home). Empower the primary caregiver (usually the baby’s family) by supplying them with information about the child’s medications (e.g. where to obtain further supplies from). Aim to make the transition as smooth as possible for the family (e.g. by contacting the pharmacist who will be taking over the care of the baby in another hospital/community setting to let them know whether there is anything in particular they need to be aware of about the baby’s medications).
- 1.Born Too Soon: The Global Action Report on Preterm Birth. March of Dimes. 2012. Accessed April 2025. https://www.efcni.org/wp-content/uploads/2018/03/Born_too_Soon.pdf
- 2.Preterm birth. World Health Organization. 2018. Accessed April 2025. https://www.who.int/news-room/fact-sheets/detail/preterm-birth
- 3.Perinatal management of extreme preterm birth before 27 weeks of gestation. British Association of Perinatal Medicine. 2019. Accessed April 2025. https://www.bapm.org/resources/80-perinatal-management-of-extreme-preterm-birth-before-27-weeks-of-gestation-2019
- 4.Elkharashi A, Vayalakkad A, Simpson A, Bowden L. The smallest ever surviving baby in the UK. Infant Journal . 2021. Accessed April 2025. https://www.infantjournal.co.uk/pdf/inf_097_7195.pdf
- 5.Low birth weight. In: Nutrition Landscape Information System. World Health Organization. 2022. Accessed April 2025. https://www.who.int/data/nutrition/nlis/info/low-birth-weight
- 6.Kalia YN, Nonato LB, Lund CH, Guy RH. Development of Skin Barrier Function in Premature Infants. Journal of Investigative Dermatology. 1998;111(2):320-326. doi:10.1046/j.1523-1747.1998.00289.x
- 7.Fluhr JW, Darlenski R, Taieb A, et al. Functional skin adaptation in infancy – almost complete but not fully competent. Experimental Dermatology. 2010;19(6):483-492. doi:10.1111/j.1600-0625.2009.01023.x
- 8.Glass L, Valdez A. Preterm Infant Incubator Humidity Levels. Advances in Neonatal Care. 2020;21(4):297-307. doi:10.1097/anc.0000000000000791
- 9.Oranges T, Dini V, Romanelli M. Skin Physiology of the Neonate and Infant: Clinical Implications. Advances in Wound Care. 2015;4(10):587-595. doi:10.1089/wound.2015.0642
- 10.Irving V, Bethell E, Burton F. Neonatal wound care: minimising trauma and pain. Wounds UK. 2006;2(1):33. https://wounds-uk.com/wp-content/uploads/2023/02/content_9046.pdf
- 11.Benitez O, Mendez M. statpearls. Published online July 3, 2023. http://www.ncbi.nlm.nih.gov/books/NBK559067/
- 12.Premature infant skin and care. DermNet. 2020. Accessed April 2025. https://dermnetnz.org/topics/premature-infant-skin-and-care
- 13.Schachner L, Andriessen A, Benjamin L, et al. The Importance of Skincare for Neonates and Infants: An Algorithm. JDD. 2021;20(11):1195-1205. doi:10.36849/jdd.6219
- 14.Boos MD, Sidbury R. Infections of the Skin. Avery’s Diseases of the Newborn. Published online 2018:1495-1502.e2. doi:10.1016/b978-0-323-40139-5.00105-4
- 15.Telofski LS, Morello AP, Mack Correa MC, Stamatas GN. The Infant Skin Barrier: Can We Preserve, Protect, and Enhance the Barrier? Dermatology Research and Practice. 2012;2012:1-18. doi:10.1155/2012/198789
- 16.Roychoudhury S, Yusuf K. Thermoregulation: Advances in Preterm Infants. NeoReviews. 2017;18(12):e692-e702. doi:10.1542/neo.18-12-e692
- 17.de Meza T. Should we use olive oil or sunflower oil on a preterm infant’s skin. Infant. 2013;9(5):170-172. https://www.infantjournal.co.uk/journal_article.html?id=6599
- 18.Oliver L, Azpeitia P, Alfonso S, et al. [Neonatal hypothyroidism secondary to the use of povidone-iodine]. Cir Pediatr. 1989;2(4):168-171. https://www.ncbi.nlm.nih.gov/pubmed/2488074
- 19.Raval RC, Singh K, Gandhi AV. Topical Therapy in Neonates. Journal of Neonatology. 2008;22(1):60-64. doi:10.1177/097321790802200114
- 20.Sharma A, Kulkarni S, Thukral A, et al. Aqueous chlorhexidine 1% versus 2% for neonatal skin antisepsis: a randomised non-inferiority trial. Arch Dis Child Fetal Neonatal Ed. 2021;106(6):643-648. doi:10.1136/archdischild-2020-321174
- 21.Chlorhexidine solutions: risk of chemical burn injury to skin in premature infants. Medicines and Healthcare products Regulatory Agency. 2015. Accessed April 2025. https://assets.publishing.service.gov.uk/media/54db105040f0b670f400000b/Chlorhexidine_DHPC_sent_29_Jan_2015.pdf
- 22.Review of chlorhexidine for skin decontamination in neonates. Neonatal and Paediatric Pharmacists Group. 2021. Accessed April 2025. https://nppg.org.uk/wp-content/uploads/2021/04/Newsletter-71-Spring-2021-web.pdf
- 23.Rehman S, Bacha D. statpearls. Published online August 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK544372/
- 24.Antenatal corticosteroids to reduce neonatal morbidity and mortality (Green-top Guideline No. 74). Royal College of Obstetricians and Gynaecologists. 2022. Accessed April 2025. https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/antenatal-corticosteroids-to-reduce-neonatal-morbidity-and-mortality-green-top-guideline-no-74/
- 25.Agarwal R, Agrawal R. Exploring Risk Factors and Perinatal Outcomes of Preterm Birth in a Tertiary Care Hospital: A Comprehensive Analysis. Cureus. Published online February 5, 2024. doi:10.7759/cureus.53673
- 26.McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews. 2020;2021(2). doi:10.1002/14651858.cd004454.pub4
- 27.King RJ. Pulmonary surfactant. Journal of Applied Physiology. 1982;53(1):1-8. doi:10.1152/jappl.1982.53.1.1
- 28.Ainsworth SB, Milligan DWA. Surfactant Therapy for Respiratory Distress Syndrome in Premature Neonates. Am J Respir Med. 2002;1(6):417-433. doi:10.1007/bf03257169
- 29.Chakraborty M, Kotecha S. Pulmonary surfactant in newborn infants and children. Breathe. 2013;9(6):476-488. doi:10.1183/20734735.006513
- 30.Verlato G, Cogo PE, Balzani M, et al. Surfactant Status in Preterm Neonates Recovering From Respiratory Distress Syndrome. Pediatrics. 2008;122(1):102-108. doi:10.1542/peds.2007-1021
- 31.Williams EE, Greenough A. Lung Protection During Mechanical Ventilation in the Premature Infant. Clinics in Perinatology. 2021;48(4):869-880. doi:10.1016/j.clp.2021.08.006
- 32.Ng EH, Shah V. Guidelines for surfactant replacement therapy in neonates. Paediatrics & Child Health. 2021;26(1):35-41. doi:10.1093/pch/pxaa116
- 33.Poractant alfa. British National Formulary . Accessed April 2025. https://bnfc.nice.org.uk/drugs/poractant-alfa/
- 34.Herting E, Härtel C, Göpel W. Less invasive surfactant administration: best practices and unanswered questions. Current Opinion in Pediatrics. 2020;32(2):228-234. doi:10.1097/mop.0000000000000878
- 35.Specialist neonatal respiratory care for babies born preterm. National Institute for Health and Care Excellence. 2020. Accessed April 2025. https://www.nice.org.uk/guidance/qs193
- 36.Mahmoud RA, Roehr CC, Schmalisch G. Current methods of non-invasive ventilatory support for neonates. Paediatric Respiratory Reviews. 2011;12(3):196-205. doi:10.1016/j.prrv.2010.12.001
- 37.Kondamudi N, Krata L, Wilt A. statpearls. Published online August 12, 2023. http://www.ncbi.nlm.nih.gov/books/NBK441969/
- 38.Pergolizzi JV Jr, Fort P, Miller TL, LeQuang JA, Raffa RB. The epidemiology of apnoea of prematurity. Clinical Pharmacy Therapeu. 2022;47(5):685-693. doi:10.1111/jcpt.13587
- 39.Abdel-Hady H. Caffeine therapy in preterm infants. WJCP. 2015;4(4):81. doi:10.5409/wjcp.v4.i4.81
- 40.Caffeine Citrate. British National Formulary for Children. Accessed April 2025. https://bnfc.nice.org.uk/drugs/caffeine-citrate/#:~:text=Loading%20dose%2020%20mg%2Fkg,to%20ensure%20that%20a%20safe
- 41.Henderson-Smart DJ, Steer PA. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database of Systematic Reviews. 2010;2013(3). doi:10.1002/14651858.cd000273.pub2
- 42.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2015;192(2):134-156. doi:10.1164/rccm.201412-2142pp
- 43.Reducing the incidence of bronchopulmonary dysplasia. British Association of Perinatal Medicine. 2023. Accessed April 2025. https://hubble-live-assets.s3.eu-west-1.amazonaws.com/bapm/file_asset/file/2310/BPD_Toolkit_Dec_2023.pdf
- 44.Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ. Published online October 20, 2021:n1974. doi:10.1136/bmj.n1974
- 45.Dankhara N, Holla I, Ramarao S, Kalikkot Thekkeveedu R. Bronchopulmonary Dysplasia: Pathogenesis and Pathophysiology. JCM. 2023;12(13):4207. doi:10.3390/jcm12134207
- 46.Baud O, Maury L, Lebail F, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. The Lancet. 2016;387(10030):1827-1836. doi:10.1016/s0140-6736(16)00202-6
- 47.Htun ZT, Schulz EV, Desai RK, et al. Postnatal steroid management in preterm infants with evolving bronchopulmonary dysplasia. J Perinatol. 2021;41(8):1783-1796. doi:10.1038/s41372-021-01083-w
- 48.Bulbul A. Role of Postnatal Corticosteroids in the Treatment or Prevention of Bronchopulmonary Dysplasia. Sisli Etfal. Published online 2023. doi:10.14744/semb.2023.80688
- 49.Specialist neonatal respiratory care for babies born preterm. NICE guideline Reference number: NG124. National Institute for Health and Care Excellence. 2019. Accessed April 2025. https://www.nice.org.uk/guidance/ng124/chapter/recommendations
- 50.Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB. Low-Dose Dexamethasone Facilitates Extubation Among Chronically Ventilator-Dependent Infants: A Multicenter, International, Randomized, Controlled Trial. Pediatrics. 2006;117(1):75-83. doi:10.1542/peds.2004-2843
- 51.Rocha G, Calejo R, Arnet V, et al. The use of two or more courses of low-dose systemic dexamethasone to extubate ventilator-dependent preterm neonates may be associated with a higher prevalence of cerebral palsy at two years of corrected age. Early Human Development. 2024;194:106050. doi:10.1016/j.earlhumdev.2024.106050
- 52.Nguyen T, Jordan BK. Let’s Talk about Dex: When do the Benefits of Dexamethasone for Prevention of Bronchopulmonary Dysplasia Outweigh the Risks? Newborn. 2022;1(1):91-96. doi:10.5005/jp-journals-11002-0009
- 53.Vrselja A, Pillow JJ, Black MJ. Effect of Preterm Birth on Cardiac and Cardiomyocyte Growth and the Consequences of Antenatal and Postnatal Glucocorticoid Treatment. JCM. 2021;10(17):3896. doi:10.3390/jcm10173896
- 54.Dice JE, Bhatia J. Patent Ductus Arteriosus: An Overview. The Journal of Pediatric Pharmacology and Therapeutics. 2007;12(3):138-146. doi:10.5863/1551-6776-12.3.138
- 55.Al-Turkait A, Szatkowski L, Choonara I, Ojha S. Management of patent ductus arteriosus in very preterm infants in England and Wales: a retrospective cohort study. bmjpo. 2022;6(1):e001424. doi:10.1136/bmjpo-2022-001424
- 56.Schneider DJ, Moore JW. Patent Ductus Arteriosus. Circulation. 2006;114(17):1873-1882. doi:10.1161/circulationaha.105.592063
- 57.Doherty T, Hu A, Salik I. statpearls. Published online April 24, 2023. http://www.ncbi.nlm.nih.gov/books/NBK539840/
- 58.Hamrick SEG, Sallmon H, Rose AT, et al. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics. 2020;146(5). doi:10.1542/peds.2020-1209
- 59.Gillam-Krakauer M, Mahajan K. statpearls. Published online August 8, 2023. http://www.ncbi.nlm.nih.gov/books/NBK430758/
- 60.Ford B, Lara S, Park J. Heart Murmurs in Children: Evaluation and Management. Am Fam Physician. 2022;105(3):250-261. https://www.ncbi.nlm.nih.gov/pubmed/35289571
- 61.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database of Systematic Reviews. 2020;2020(2). doi:10.1002/14651858.cd003481.pub8
- 62.Mitra S, Florez ID, Tamayo ME, et al. Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen With Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants. JAMA. 2018;319(12):1221. doi:10.1001/jama.2018.1896
- 63.King E, Horn D, Gluchowski N, O’Reilly D, Fiander M, Soll R. Safety and efficacy of proton pump inhibitors in preterm infants with gastroesophageal reflux disease. Cochrane Database of Systematic Reviews. 2023;2023(5). doi:10.1002/14651858.cd015127
- 64.Jasani B, Mitra S, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database of Systematic Reviews. 2022;2022(12). doi:10.1002/14651858.cd010061.pub5
- 65.García-Robles A, Gimeno Navarro A, Serrano Martín M del M, et al. Paracetamol vs. Ibuprofen in Preterm Infants With Hemodynamically Significant Patent Ductus Arteriosus: A Non-inferiority Randomized Clinical Trial Protocol. Front Pediatr. 2020;8. doi:10.3389/fped.2020.00372
- 66.McNamara P, Weisz D. Patent ductus arteriosus ligation and adverse outcomes: Causality or bias? J Clin Neonatol. 2014;3(2):67. doi:10.4103/2249-4847.134670
- 67.Roberts P, Adwani S, Archer N, Wilson N. Catheter closure of the arterial duct in preterm infants. Archives of Disease in Childhood – Fetal and Neonatal Edition. 2007;92(4):F248-F250. doi:10.1136/adc.2005.078600
- 68.Ping P, Yu B, Xu R, Zhao P, He S. Monitoring and evaluation of hypotension in the extremely preterm. Front Cardiovasc Med. 2024;11. doi:10.3389/fcvm.2024.1477337
- 69.Dilli D, Soylu H, Tekin N. Turkish Neonatal Society guideline on the neonatal hemodynamics and management of hypotension in newborns. Turk Pediatri Ars. 2019;53(sup1):65-75. doi:10.5152/turkpediatriars.2018.01801
- 70.Agakidou E, Chatziioannidis I, Kontou A, Stathopoulou T, Chotas W, Sarafidis K. An Update on Pharmacologic Management of Neonatal Hypotension: When, Why, and Which Medication. Children. 2024;11(4):490. doi:10.3390/children11040490
- 71.Hwang-Bo S, Seo YM, Oh MY, Im SA, Youn YA. The prognosis of refractory hypotension and severe intraventricular hemorrhage in very low birth weight infants. Medicine. 2022;101(30):e29598. doi:10.1097/md.0000000000029598
- 72.Mullaly R, El-Khuffash AF. Haemodynamic assessment and management of hypotension in the preterm. Arch Dis Child Fetal Neonatal Ed. 2023;109(2):120-127. doi:10.1136/archdischild-2022-324935
- 73.Iijima S. Late-onset glucocorticoid-responsive circulatory collapse in premature infants. Pediatrics & Neonatology. 2019;60(6):603-610. doi:10.1016/j.pedneo.2019.09.005
- 74.Dasgupta S, Jain S, Aly A. Neonatal Hypotension, the Role of Hydrocortisone and Other Pharmacological Agents in its Management. J Pediatr Child Care. 2016. Accessed April 2025. https://www.avensonline.org/fulltextarticles/jpcc-2380-0534-02-0014.html#:~:text=Although%20the%20addition%20of%20hydrocortisone,and%20should%20be%20weaned%20gradually
- 75.Woodgate P, Jardine L. Neonatal jaundice. BMJ Clin Evid. 2011;2011. https://www.ncbi.nlm.nih.gov/pubmed/21920055
- 76.Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med. 2017;78(12):699-704. doi:10.12968/hmed.2017.78.12.699
- 77.Newborn jaundice. NHS. 2022. Accessed April 2025. https://www.nhs.uk/conditions/jaundice-newborn/
- 78.Reddy D, Pandey S. statpearls. Published online June 25, 2023. http://www.ncbi.nlm.nih.gov/books/NBK559120/
- 79.Jaundice in newborn babies under 28 days. National Institute for Health and Care Excellence. 2023. Accessed April 2025. https://www.nice.org.uk/guidance/cg98/evidence/full-guideline-245411821
- 80.Wang J, Guo G, Li A, Cai WQ, Wang X. Challenges of phototherapy for neonatal hyperbilirubinemia (Review). Exp Ther Med. 2021;21(3). doi:10.3892/etm.2021.9662
- 81.Woodgate P, Jardine L. Neonatal jaundice: phototherapy. BMJ Clin Evid. 2015;2015. https://www.ncbi.nlm.nih.gov/pubmed/25998618
- 82.Pan J, Zhan C, Yuan T, et al. Intravenous immunoglobulin G in the treatment of ABO hemolytic disease of the newborn during the early neonatal period at a tertiary academic hospital: a retrospective study. J Perinatol. 2021;41(6):1397-1402. doi:10.1038/s41372-021-00963-5
- 83.Wolf MF, Childers J, Gray KD, et al. Exchange transfusion safety and outcomes in neonatal hyperbilirubinemia. J Perinatol. 2020;40(10):1506-1512. doi:10.1038/s41372-020-0642-0
- 84.Hakeem AbdelHAA, Mohamed G, Othman M. Retinopathy of prematurity: A study of prevalence and risk factors. Middle East Afr J Ophthalmol. 2012;19(3):289. doi:10.4103/0974-9233.97927
- 85.Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. The Lancet. 2013;382(9902):1445-1457. doi:10.1016/s0140-6736(13)60178-6
- 86.Chen J, Smith LEH. Retinopathy of prematurity. Angiogenesis. 2007;10(2):133-140. doi:10.1007/s10456-007-9066-0
- 87.Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Survey of Ophthalmology. 2018;63(5):618-637. doi:10.1016/j.survophthal.2018.04.002
- 88.Hartnett ME, Penn JS. Mechanisms and Management of Retinopathy of Prematurity. N Engl J Med. 2012;367(26):2515-2526. doi:10.1056/nejmra1208129
- 89.UK Screening of Retinopathy of Prematurity Guideline. Royal College of Paediatrics and Child Health in collaboration with Royal College of Ophthalmologists and British Association of Perinatal Medicine. 2024. Accessed April 2025. https://www.rcpch.ac.uk/sites/default/files/2024-10/rop-screening-guideline-full-2022_updated-2024.pdf
- 90.Sen P, Jain S, Bhende P. Stage 5 retinopathy of prematurity: An update. Taiwan J Ophthalmol. 2018;8(4):205. doi:10.4103/tjo.tjo_61_18
- 91.Senjam S, Chandra P. Retinopathy of prematurity: Addressing the emerging burden in developing countries. J Family Med Prim Care. 2020;9(6):2600. doi:10.4103/jfmpc.jfmpc_110_20
- 92.Barnett JM, Hubbard GB. Complications of retinopathy of prematurity treatment. Current Opinion in Ophthalmology. 2021;32(5):475-481. doi:10.1097/icu.0000000000000783
- 93.Alzuabi AK, Alshammari OM, Almousa AN, Abouammoh MA. Anti-vascular endothelial growth factor therapy in retinopathy of prematurity. Saudi Journal of Ophthalmology. 2022;36(3):260-269. doi:10.4103/sjopt.sjopt_12_22
- 94.Clinical Commissioning Policy: Ranibizumab in Retinopathy of Prematurity . NHS England. 2023. Accessed April 2025. https://www.england.nhs.uk/wp-content/uploads/2023/05/2201-ranibizumab-in-retinopathy-of-prematurity-policy.pdf
- 95.Treating Retinopathy of Prematurity in the UK. Royal College of Ophthalmologists. 2022. Accessed April 2025. https://www.rcophth.ac.uk/wp-content/uploads/2022/03/Treating-Retinopathy-of-Prematurity-in-the-UK-Guideline-Exec-Summary.pdf
- 96.Tawfik GM, Shahein EA, Dabour SA, Hassanein D, Elshewy AM. Comparison of intravitreal injection of ranibizumab versus bevacizumab for treatment of type 1 and aggressive retinopathy of prematurity in rural Egypt. A randomized clinical trial. BMJ Open Ophth. 2022;7(1):e001173. doi:10.1136/bmjophth-2022-001173
- 97.Bouyssi-Kobar M, du Plessis AJ, McCarter R, et al. Third Trimester Brain Growth in Preterm Infants Compared With In Utero Healthy Fetuses. Pediatrics. 2016;138(5):e20161640. doi:10.1542/peds.2016-1640
- 98.Ballabh P. Intraventricular Hemorrhage in Premature Infants: Mechanism of Disease. Pediatr Res. 2010;67(1):1-8. doi:10.1203/pdr.0b013e3181c1b176
- 99.Grotheer M, Bloom D, Kruper J, et al. Human white matter myelinates faster in utero than ex utero. Proc Natl Acad Sci USA. 2023;120(33). doi:10.1073/pnas.2303491120
- 100.SHANG Q, MA CY, LV N, et al. Clinical study of cerebral palsy in 408 children with periventricular leukomalacia. Experimental and Therapeutic Medicine. 2015;9(4):1336-1344. doi:10.3892/etm.2015.2222
- 101.Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. The Lancet Infectious Diseases. 2014;14(8):751-762. doi:10.1016/s1473-3099(14)70710-8
- 102.Silveira R, Corso A, Procianoy R. The Influence of Early Nutrition on Neurodevelopmental Outcomes in Preterm Infants. Nutrients. 2023;15(21):4644. doi:10.3390/nu15214644
- 103.Mulkey SB, du Plessis AJ. The Immature Autonomic Nervous System, Hemodynamic Regulation, and Brain Injury in the Preterm Neonate. Hemodynamics and Cardiology. Published online 2019:111-127. doi:10.1016/b978-0-323-53366-9.00007-7
- 104.Mitha A, Chen R, Razaz N, et al. Neurological development in children born moderately or late preterm: national cohort study. BMJ. Published online January 24, 2024:e075630. doi:10.1136/bmj-2023-075630
- 105.Chung EH, Chou J, Brown KA. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr. 2020;9(S1):S3-S8. doi:10.21037/tp.2019.09.10
- 106.Panayiotopoulos C. Neonatal Seizures and Neonatal Syndromes. In: The Epilepsies: Seizures, Syndromes and Management. Bladon Medical Publishing; 2005. Accessed April 2025. https://www.ncbi.nlm.nih.gov/books/NBK2599/
- 107.Silverstein FS, Jensen FE. Neonatal seizures. Annals of Neurology. 2007;62(2):112-120. doi:10.1002/ana.21167
- 108.Zupanc ML. Neonatal seizures. Pediatric Clinics of North America. 2004;51(4):961-978. doi:10.1016/j.pcl.2004.03.002
- 109.Seizures in the neonate. NHS Greater Glasgow and Clyde Paediatrics for Health Professionals. 2022. Accessed April 2025. https://www.clinicalguidelines.scot.nhs.uk/nhsggc-guidelines/nhsggc-guidelines/neonatology/seizures-in-the-neonate/
- 110.van Rooij LGM, Hellström-Westas L, de Vries LS. Treatment of neonatal seizures. Seminars in Fetal and Neonatal Medicine. 2013;18(4):209-215. doi:10.1016/j.siny.2013.01.001
- 111.Slaughter LA, Patel AD, Slaughter JL. Pharmacological Treatment of Neonatal Seizures. J Child Neurol. 2013;28(3):351-364. doi:10.1177/0883073812470734
- 112.Mruk AL, Garlitz KL, Leung NR. Levetiracetam in Neonatal Seizures: A Review. The Journal of Pediatric Pharmacology and Therapeutics. 2015;20(2):76-89. doi:10.5863/1551-6776-20.2.76
- 113.Shaker H, Al-Amrani F, Al Mandhari H. Case Series of Midazolam-Induced Seizures-Like Activity in Five Neonates. Sultan Qaboos Univ Med J. 2024;24(3):394-398. doi:10.18295/squmj.10.2023.063
- 114.Ahmed M, Raj D, Upadhyay N. Midazolam Induced Abnormal Movements in Preterm Newborns: Case Series. CEJP. 2022;18(2):123. doi:10.5457/p2005-114.325
- 115.Almannai M, Al Mahmoud RA, Mekki M, El-Hattab AW. Metabolic Seizures. Front Neurol. 2021;12. doi:10.3389/fneur.2021.640371
- 116.Glass HC. Neonatal Seizures. Clinics in Perinatology. 2014;41(1):177-190. doi:10.1016/j.clp.2013.10.004
- 117.Favié LMA, Huitema ADR, van den Broek MPH, et al. Lidocaine as treatment for neonatal seizures: Evaluation of previously developed population pharmacokinetic models and dosing regimen. Brit J Clinical Pharma. 2020;86(1):75-84. doi:10.1111/bcp.14136
- 118.Chinoy A, Mughal MZ, Padidela R. Metabolic bone disease of prematurity: causes, recognition, prevention, treatment and long-term consequences. Arch Dis Child Fetal Neonatal Ed. 2019;104(5):F560-F566. doi:10.1136/archdischild-2018-316330
- 119.Faienza MF, D’Amato E, Natale MP, et al. Metabolic Bone Disease of Prematurity: Diagnosis and Management. Front Pediatr. 2019;7. doi:10.3389/fped.2019.00143
- 120.Embleton ND, Jennifer Moltu S, Lapillonne A, et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper From the ESPGHAN Committee on Nutrition and Invited Experts. Journal of Pediatric Gastroenterology & Nutrition. 2022;76(2):248-268. doi:10.1097/mpg.0000000000003642
- 121.The routine supplementation of vitamins and iron and the management of zinc deficiency in preterm and small for gestational age infants. Neonatal Dietitians Group (NDiG) – BDA. 2024. Accessed April 2025. https://www.bda.uk.com/static/eb595771-f10f-417a-966988888f2d48de/NDiG-Vit-iron-zinc-October-2024-FINAL-V14.pdf
- 122.Marino LV, Pearson F, Johnson MJ. Specialised milk feeds for term and preterm infants. Infant. 2018;14(4). https://www.infantjournal.co.uk/pdf/inf_082_cia.pdf
- 123.Grover M, Ashraf AP, Bowden SA, et al. Invited Mini Review Metabolic Bone Disease of Prematurity: Overview and Practice Recommendations. Horm Res Paediatr. Published online January 11, 2024:1-11. doi:10.1159/000536228
- 124.Forster C, Hoodbhoy S, Macdougall C, et al. Practical approach to managing metabolic bone disease of prematurity in the neonatal unit. Arch Dis Child Educ Pract Ed. 2023;109(3):143-146. doi:10.1136/archdischild-2023-326100