Shutterstock.com
After reading this learning article, you should be able to:
- Understand the causes and risk factors for developing primary biliary cholangitis (PBC);
- Identify the symptoms of PBC and know how the disease is diagnosed;
- Understand the treatment options available and how symptoms are managed.
Primary biliary cholangitis (PBC), formerly known as primary biliary cirrhosis, is a progressive autoimmune cholestatic liver disease that results in end-stage liver disease and the need for liver transplantation if left untreated[1].
A UK study involving 770 individuals with definite or probable PBC demonstrated a prevalence of 35 cases per 100,000 and an annual incidence of 2–3 cases per 100,000[1–3]. Currently, there are an estimated 20,000 patients living with PBC in the UK[2]. The median age at diagnosis is 65 years[1]. Women are predominantly affected by PBC; 9 out of 10 patients diagnosed are women and the prevalence can be as high as 1 in 800 in women aged over 45 years[1,4,5]. The reason for the disproportionate frequency in women is currently unknown.
Several large case-control cohort studies have demonstrated links between PBC and recurrent urinary tract infections, reproductive hormone replacement, use of nail polish and cigarette smoking[6–8]. These studies have been bolstered by identification of disease clusters, where the prevalence in certain areas with infectious or environmental exposure to toxins is higher than the general population[9]. Anti-mitochondrial autoantibodies (AMA) from PBC-positive patients have been shown to strongly cross-react with xenobiotics that mimic or modify lipoic acid[10]. Examples of these xenobiotics are 2-octynoic acid, which is commonly found in cosmetic products, perfumes and detergents, and 6,8-bis(acetylthio)octanoic acid, a metabolite of paracetamol[10]. It is thought that some individuals are genetically predisposed to PBC[11–13].
Patients with PBC have a higher mortality rate than that of the general population[1,14,15]. Left untreated, PBC carries an average survival of 9–10 years from presentation; around 25% of patients develop liver failure during this time[15]. Independent risk factors for poorer prognosis include age, hepatomegaly, and advanced fibrosis and cirrhosis[1]. Raised serum bilirubin levels are associated with more advanced liver disease and thus a poorer prognosis[5].
Carrying a poor prognosis if left untreated, PBC is associated with a high symptom burden that can affect the patient’s quality of life (QoL)[1,16,17]. The clinical course of PBC is highly variable; it is therefore imperative that treatment is personalised. There is an opportunity for pharmacist involvement in ensuring treatment and supportive therapies are optimised; a multidisciplinary approach should be adopted to maximise patient outcomes.
Pathophysiology
PBC is the progressive immune-mediated destruction of the bile ducts. Gradual loss of bile ducts (i.e. ductopenia) leads to a reduction in bile flow (i.e. cholestasis), causing bile to accumulate in the liver, resulting in cholestatic liver injury[11]. Over time, this cholestatic insult can lead to liver fibrosis and eventually cirrhosis[2].
Clinical presentation
The symptoms of PBC are often non-specific, and some patients are asymptomatic in the early stages of the disease[1]. Symptoms of early and advanced PBC are outlined in Table 1[16,17]. Fatigue is the most common symptom, reported by 50% to 78% of patients[5]. Fatigue greatly impacts the patient’s QoL, and may be associated with orthostatic hypotension, daytime somnolence, cognitive defects and slow muscle recovery following exercise[5]. The underlying aetiology of fatigue in PBC is unknown[5].
Pruritus (itch) is reported in 20% to 70% of patients and tends to be worse at night[5]. The severity of pruritus can fluctuate, and does not correlate with severity of liver disease[5]. Pruritus in PBC is unexplained; however, links to autotaxin enzyme activity, accumulation of bile components in serum, and increased concentrations of endogenous opioids have been suggested[5,14].
Table 1: Signs and symptoms of primary biliary cholangitis[4,16,17]
| Early disease | Advanced disease |
| Fatigue; Pruritus; Right upper quadrant pain; Orthostatic hypotension; Cognitive defects (poor concentration/memory); Daytime somnolence (sleepiness); Dry eyes; Dry mouth; Vaginal dryness; Bone and joint aches; Hyperpigmentation; Xanthomas (fatty deposits in skin). | Jaundice; Spider naevi; Ascites; Hepatic encephalopathy; Portal hypertension; Oesophageal varices; Hypersplenism; Osteoporosis; Sexual dysfunction; Oedema; Muscle wasting. |
Diagnosis
PBC is typically identified using routine blood tests in primary care, owing to the often asymptomatic or vague nature of clinical presentations[1]. Significantly elevated levels of alkaline phosphatase (ALP) and gamma-glutamyl transferase are characteristic of cholestasis, and are present in early PBC[1]. Elevated bilirubin and hypoalbuminaemia are features of advanced fibrosis or cirrhosis[1]. Patients with unexplained cholestatic biochemistry should be referred to secondary or tertiary hepatology or gastroenterology services for further investigation[1,15].
A probable diagnosis of PBC may be made based on the presence of serological AMA or anti-nuclear autoantibodies (ANA), which are present in 95% and 30% of patients with PBC, respectively[1]. Immunologic abnormalities are also seen: hyper-elevated levels of serum IgM are found in the majority of patients[1,5]. Elevated IgG may be observed in PBC patients with autoimmune hepatitis crossover, or in those with more advanced disease[1,9].
All patients with suspected PBC should have a baseline abdominal ultrasound scan as part of their investigation to exclude extrahepatic causes of cholestasis, and to identify any features of advanced PBC such as splenomegaly, ascites, portal hypertension and focal liver lesions[1,15]. Liver biopsy is not routinely recommended, owing to the variable nature of PBC histology confounding diagnosis[1]. Non-invasive techniques, such as transient elastography (FibroScan), are preferred for determining disease stage[1].
There is a strong association between PBC and other autoimmune conditions including Sjögren’s syndrome, autoimmune thyroid disease, CREST syndrome, scleroderma, and Raynaud’s disease[1]. A cohort study demonstrated that 61.2% of PBC patients had a comorbid autoimmune condition[18].
Differential diagnoses include primary sclerosing cholangitis, sarcoid, graft-versus-host disease (in those with a relevant history), idiopathic ductopenia, and variants of genetic cholestatic syndromes; these differentials should be considered in the absence of AMA or ANA autoantibodies[1]. Low-titre AMA may represent a false positive result, which can occur in conditions causing inflammation, particularly in non-alcohol fatty liver disease[1].
Drug-induced liver injury should also be considered as a differential diagnosis[1]. It is therefore important that the patient has a complete and thorough drug history taken, including illicit and herbal medications, covering the past three months. Particular attention should be paid to drugs that are known to elicit cholestatic injury, such as co-amoxiclav, co-trimoxazole, azathioprine and anabolic steroids[15,19].
Treatment
Treatment of PBC aims to slow disease progression and reduce the need for transplantation. Even with treatment, PBC remains a progressive disease with risk of liver-related complications and death[15].
Ursodeoxycholic acid
The first-line treatment for PBC is ursodeoxycholic acid (UDCA)[1,5,15]. UDCA is an endogenous bile acid that exerts anti-inflammatory, immunomodulatory and anti-apoptotic effects within the liver through bile enrichment and displacement of toxic hydrophobic bile acids[20].
UDCA is recommended at a dose of 13–15mg/kg/day, continued life-long[1,5,15]. A randomised controlled trial compared UDCA low dose (5–7mg/kg/day), standard dose (13–15mg/kg/day) and high dose (25mg/kg/day), and found the standard dose to be superior in biochemical response and cost-effectiveness[21].
A Cochrane review concluded that UDCA showed beneficial effects on biochemical markers, such as ALP and bilirubin; however, the review did not demonstrate any benefit or harm on all-cause mortality or liver transplantation[22]. Owing to the slow natural history of PBC, individual studies have been unable to adequately address end points such as liver transplantation and death[1]. However, a meta-analysis conducted by the Global PBC Study Group revealed significantly improved transplant-free survival in those treated with UDCA compared with untreated individuals at 5, 10 and 15 years[23]. The study concluded that ALP and bilirubin are appropriate surrogate end points for patient outcomes[23]. Lower ALP and bilirubin levels coincide with improved transplant-free survival[23].
Prognostic models, such as the GLOBE and UK PBC score, utilise age, ALP, bilirubin, aspartate transaminase/alanine transaminase (AST/ALT) (UK PBC only), albumin and platelet levels to predict mortality and need for transplantation in patients being treated with UDCA[1,5,15]. These scores can guide escalation of medical therapy and stratification for liver transplantation[1,5,15].
Around 40% of patients do not respond to UDCA[23]. Non-response to UDCA is defined as ALP > 1.67 x upper limit of normal (ULN) and/or bilirubin > ULN (but less than 2 x ULN) after one year of treatment[1,5,15]. A second-line option should be added in these patients[1,5,15]. Second-line monotherapy is only recommended for those unable to tolerate UDCA[1,5,15].
UDCA is generally well-tolerated, but side effects include weight gain in the first 12 months, gastrointestinal (GI) intolerance and hair loss[24]. Patients should be advised that this weight gain is not progressive, but will persist over the course of treatment[1,5,25]. GI intolerance of UDCA may be improved by splitting the dose over the day, or may require dose reduction[1,24].
Obeticholic acid
A licensed second-line oral therapy is obeticholic acid (OCA), which modulates bile acid synthesis, and increases transport of bile acids out of hepatocytes, limiting hepatic exposure[26–28]. The PBC OCA International Study of Efficacy (POISE) trial found that OCA was effective in reducing ALP and bilirubin (surrogate markers for survival), compared with placebo, in patients who did not respond to UDCA monotherapy[27].
The most common side effects of OCA are abdominal pain, pruritus and fatigue[26]. Patients should discuss these issues with their doctor, pharmacist or nurse specialist so that they can be managed appropriately. As per NHS England commissioning requirements, OCA may only be supplied by a specialist centre with a designated PBC multidisciplinary team[29]. OCA is a high-cost drug so it is important that it is prescribed appropriately in those who would benefit the most[29].
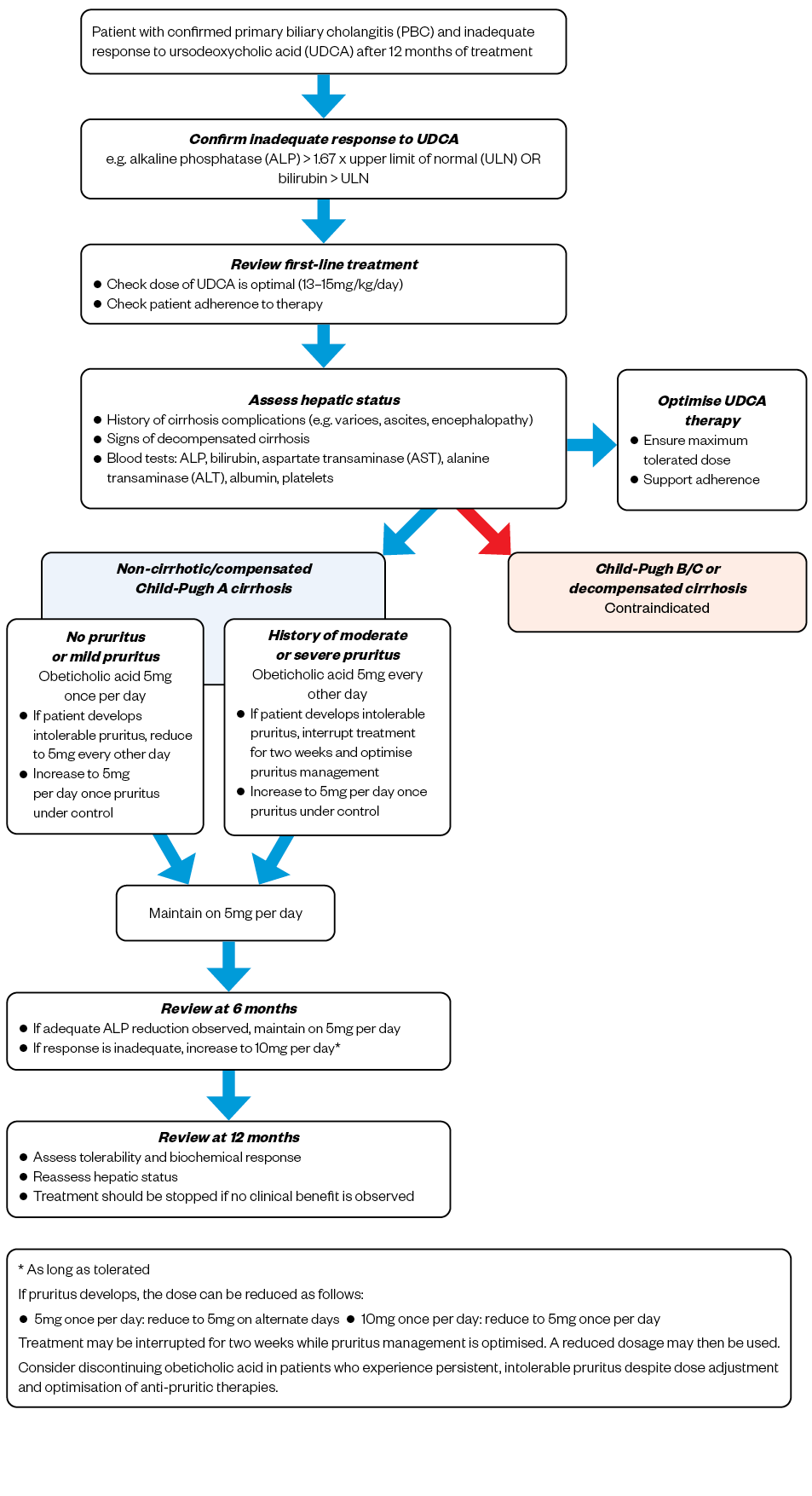
Hepatic failure has been reported with OCA in patients with compensated or decompensated cirrhosis. OCA is contraindicated in those with Child-Pugh B or C cirrhosis, and those who have had a prior decompensation event. Patients who are at an increased risk of decompensation, such as those with signs of portal hypertension; for example, ascites, gastroesophageal varices, persistent thrombocytopenia, or patients with concomitant liver disease and/or severe intercurrent illness, should be monitored more closely. OCA should be permanently discontinued in any patients displaying signs or symptoms of hepatic decompensation, or who progress to Child-Pugh B/C cirrhosis[26]. Figure 1 shows a suggested algorithm for OCA treatment[26,30].
OCA may lead to reduced international normalised ratio (INR) in patients taking warfarin, and therefore INR should be monitored, and the dose of warfarin adjusted when taken concomitantly[26]. OCA may also increase the exposure to CYP1A2 substrates, so therapeutic monitoring is recommended for CYP1A2 substrates with a narrow therapeutic index, such as theophylline or tizanidine[26].
Fibrates
Fibrates (e.g. bezafibrate) are an unlicensed treatment for PBC that may be considered in those unresponsive or intolerant to OCA[1]. Fibrates exert potent anticholestatic effects through activation of peroxisome proliferator-activated receptors, and downregulate several pathways involved in bile acid synthesis[1]. A randomised controlled trial demonstrated that bezafibrate at a dose of 400mg orally per day reduced ALP and improved pruritus symptoms[31]. Fibrates require dose reduction in renal impairment owing to the risk of nephrotoxicity; therefore, renal function requires close monitoring during treatment[32,33].
Budesonide
Off-label budesonide has also been used in PBC, with trials using doses from 6–9mg orally per day[1,15]. Budesonide is a synthetic corticosteroid that undergoes high first-pass metabolism within the liver, thus reducing systemic side effects compared with other steroids[15]. Care must be taken in advanced liver disease, where this first-pass effect is diminished owing to reduced metabolic function[15]. Budesonide does not currently feature in UK or European guidelines for treatment of PBC; however, there are Phase III trials under way[1,15].
Liver transplantation
Patients who meet minimal listing criteria using the United Kingdom Model for End-Stage Liver Disease (UKELD) score may be considered for liver transplantation. The UKELD score is calculated from bilirubin, creatinine, INR and sodium levels[34]. Intractable pruritus that has not responded to therapy is also an indication for transplantation in the absence of an elevated UKELD score[1]. Owing to increased awareness and earlier diagnosis, as well as the introduction of effective medical treatments, the number of liver transplantations for PBC in Europe and North America is falling[5].
Management of symptoms
Fatigue
There is currently no licensed therapy for the management of fatigue, despite it greatly impacting patient QoL[35,36]. Ondansetron, fluoxetine and modafinil have all been investigated as possible treatment options, but have failed to show benefit[5,37–39]. Underlying comorbidities that cause fatigue, such as hypothyroidism, depression and anaemia, should be considered and treated[1,5]. Patients should be counselled on lifestyle changes, such as taking regular breaks and engaging in gentle daily aerobic exercise[40].
Pruritus
Any part of the body can be affected by pruritus, but the palms of the hands and soles of the feet are commonly involved[40]. The intensity of pruritus tends to worsen overnight, affecting sleep and contributing to daytime somnolence[40].
The first-line pharmacological treatment for pruritus is colestyramine, a non-absorbable bile resin[1,41]. Patients should be advised that colestyramine must be administered one hour after or four hours before other oral medication, as it can affect their absorption[42]. For patients taking OCA, a minimum four-hour window is recommended between doses of OCA and colestyramine[26]. This interaction with other medication appears to be the result of two mechanisms: binding of colestyramine to the drug itself in the gut (e.g. in the case of warfarin), and reduction in enterohepatic circulation owing to reduced reabsorption caused by increased bile excretion[42,43]. Increased monitoring is recommended for critical medicines, such as warfarin and levothyroxine[42,43]. Supplementation of fat-soluble vitamins (vitamins A, D, K and E) may be required if high doses are used long-term[42]. Colestyramine is generally well tolerated, but patients may experience GI side effects such as constipation and GI discomfort[42]. These side effects can be managed by increasing fibre intake, and taking over-the-counter laxatives[42].
Rifampicin is a second-line option that has demonstrated benefit in several prospective randomised controlled trials[44–47]. Doses range from 150mg per day to 300mg twice per day[44–48]. Regular blood tests are required, as rifampicin is associated with hepatotoxicity and haemolysis[1]. Rifampicin affects vitamin K metabolism and can increase the INR, especially in icteric patients; therefore, rifampicin should be avoided in patients with hyperbilirubinaemia[5]. Rifampicin is an enzyme inducer, so drug–drug interactions need to be considered, and the patient should be advised to speak to a pharmacist prior to starting new medication[5,48].
Opiate antagonists, such as naltrexone, are increasingly being used as third-line agents, as they reduce the sensation of itching and subsequent scratching behaviour[1,49]. Opiate antagonists can lead to opiate withdrawal-like reactions (including abdominal pain, tachycardia, goosebumps and nightmares) in the first few days of treatment; therefore, it is recommended to start naltrexone at a low dose of 12.5mg per day, increasing by 12.5mg every three to seven days until pruritus is alleviated[5]. Ongoing withdrawal sensations and reduced pain threshold may affect long-term tolerability[1,5]. Opiate antagonists should be used with caution in those taking opioids, so a thorough drug history should be compiled and regularly reviewed[50].
Although PBC-associated pruritus is not histamine-mediated, sedating antihistamines, such as chlorphenamine, may be used for their sedative properties in managing night-time pruritus[5,51]. Antihistamines have anticholinergic effects, which can worsen dry mouth[52].
Selective serotonin reuptake inhibitors (SSRIs) may be used off-label in uncontrolled pruritus[1]. Several studies have shown that sertraline, at a dose of 75–100mg per day, can relieve pruritus[5,53]. It should be noted that SSRIs can worsen dry mouth[54].
Box 1 provides a summary of over-the-counter and non-pharmacological measures that pharmacists can recommend for the management of pruritis.
Box 1: Over-the-counter and non-pharmacological measures for managing pruritus
- Regular use of moisturisers to avoid dry skin;
- Apply menthol 1% in aqueous cream (can be stored in fridge to enhance cooling effect);
- Avoid hot showers and baths;
- Wear loose-fitting clothing and underwear made from natural fibres;
- Practice relaxation techniques and mindfulness to break the itch–scratch cycle[40,51].
Sicca complex
Dry mouth (xerostomia) and dry eyes are frequently seen in PBC patients[1,5]. Artificial saliva replacement, frequent sips of water, and sugar-free gum can help alleviate symptoms of dry mouth[1,5]. Xerostomia increases the risk of dental caries, so patients should be advised on good dental hygiene[1,5]. Dry eyes can be managed with artificial tears; a muscarinic receptor agonist, such as pilocarpine, may be used if symptoms do not resolve[1,5].
As women are disproportionately affected by PBC, vaginal dryness is a symptom that pharmacists should enquire about[1,15]. Vaginal moisturisers can be used by female patients experiencing vaginal dryness[1,15]. The use of oestrogen creams should be directed by primary care or gynaecology, but there are no specific contraindications[1,15].
Osteoporosis
Some 20–44% of PBC patients develop osteoporosis[1,5,55]. Low bone mineral density is thought to occur as a result of cholestasis, and cirrhosis is an independent risk factor for development of osteoporosis[56]. Autonomic dysfunction can result in falls, increasing the likelihood of fractures; therefore, patients should undergo appropriate surveillance and monitoring. Cirrhotic patients, post-menopausal women, older patients and those with a low body mass index should have a Fracture Risk Assessment Tool (FRAX) score calculated and their bone density measured[1,55]. The FRAX score calculates the probability of a hip or major osteoporotic fracture within 10 years[57].
Calcium and vitamin D supplementation is indicated in those who do not achieve adequate dietary intake[1,5,15]. Alendronic acid is an appropriate first-line option for those who require bisphosphonate treatment[1,55,58]. Patients who are unable to tolerate oral bisphosphonates should be referred for specialist management[1]. Patients can reduce their risk of fractures through weight-bearing exercise, smoking cessation and minimising alcohol intake[15].
Best practice for pharmacists
- Primary biliary cholangitis (PBC) requires a holistic approach specifically tailored to patient needs to maximise outcomes and improve quality of life;
- Drug histories should be accurate and kept up to date to identify drug-induced liver injuries and ensure treatment is escalated appropriately;
- Regular review of patient symptoms should be undertaken to ensure symptom management is optimal;
- Pharmacists should be knowledgeable about available OTC preparations that can be used to alleviate symptoms;
- PBC treatment should be regularly reviewed for efficacy and tolerability;
- Pharmacists should be able to spot red flags for worsening liver function (e.g. development/worsening of ascites, jaundice, hepatic encephalopathy) that suggest disease progression.
This article has been reviewed by the expert author to ensure it remains relevant and up to date, following its original publication in The Pharmaceutical Journal in March 2021.
- 1Hirschfield GM, Dyson JK, Alexander GJM, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 2018;67:1568–94. doi:10.1136/gutjnl-2017-315259
- 2Epidemiology of PBC. UK-PBC. 2019.http://www.uk-pbc.com/about/aboutpbc/epidemiology-of-pbc/ (accessed May 2023).
- 3James OF, Bhopal R, Howel D, et al. Primary biliary cirrhosis once rare, now common in the United Kingdom? Hepatology 1999;30:390–4. doi:10.1002/hep.510300213
- 4Primary Biliary Cholangitis (PBC). British Liver Trust. 2023.https://britishlivertrust.org.uk/information-and-support/living-with-a-liver-condition/liver-conditions/primary-biliary-cholangitis/#:~:text=British%20Liver%20Trust%20urges%20people%20at%20risk%20of,the%20tubes%20linking%20your%20liver%20to%20your%20gut. (accessed May 2023).
- 5Lindor KD, Bowlus CL, Boyer J, et al. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology Published Online First: 6 November 2018. doi:10.1002/hep.30145
- 6Prince MI, Ducker SJ, James OFW. Case-control studies of risk factors for primary biliary cirrhosis in two United Kingdom populations. Gut 2010;59:508–12. doi:10.1136/gut.2009.184218
- 7Gershwin M, Selmi C, Worman H. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology 2005;42:1194–202. doi:10.1002/hep.20907
- 8Lammert C, Nguyen DL, Juran BD, et al. Questionnaire based assessment of risk factors for primary biliary cirrhosis. Digestive and Liver Disease 2013;45:589–94. doi:10.1016/j.dld.2013.01.028
- 9McNally RJQ, James PW, Ducker S, et al. No Rise in Incidence but Geographical Heterogeneity in the Occurrence of Primary Biliary Cirrhosis in North East England. American Journal of Epidemiology 2014;179:492–8. doi:10.1093/aje/kwt308
- 10Tanaka T, Zhang W, Sun Y, et al. Autoreactive monoclonal antibodies from patients with primary biliary cholangitis recognize environmental xenobiotics. Hepatology 2017;66:885–95. doi:10.1002/hep.29245
- 11Hirschfield GM, Gershwin ME. The Immunobiology and Pathophysiology of Primary Biliary Cirrhosis. Annu Rev Pathol Mech Dis 2013;8:303–30. doi:10.1146/annurev-pathol-020712-164014
- 12Cordell HJ, Han Y, et al. International genome-wide meta-analysis identifies new primary biliary cirrhosis risk loci and targetable pathogenic pathways. Nat Commun 2015;6. doi:10.1038/ncomms9019
- 13Selmi C, Mayo MJ, Bach N, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: Genetics, epigenetics, and environment. Gastroenterology 2004;127:485–92. doi:10.1053/j.gastro.2004.05.005
- 14Kaplan MM. Primary Biliary Cirrhosis. N Engl J Med 1996;335:1570–80. doi:10.1056/nejm199611213352107
- 15Hirschfield GM, Beuers U, Corpechot C, et al. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. Journal of Hepatology 2017;67:145–72. doi:10.1016/j.jhep.2017.03.022
- 16PBC Symptoms. PBCers Organization. 2019.https://pbcers.org/symptoms/ (accessed May 2023).
- 17American Liver Foundation. Primary Biliary Cholangitis. 2017.https://liverfoundation.org/for-patients/about-the-liver/diseases-of-the-liver/primary-biliary-cholangitis/ (accessed May 2023).
- 18Floreani A, Franceschet I, Cazzagon N, et al. Extrahepatic Autoimmune Conditions Associated with Primary Biliary Cirrhosis. Clinic Rev Allerg Immunol 2014;48:192–7. doi:10.1007/s12016-014-8427-x
- 19Hoofnagle JH, Björnsson ES. Drug-Induced Liver Injury — Types and Phenotypes. N Engl J Med 2019;381:264–73. doi:10.1056/nejmra1816149
- 20Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. Journal of Hepatology 2001;35:134–46. doi:10.1016/s0168-8278(01)00092-7
- 21Angulo P, Dickson ER, Therneau TM, et al. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomized trial. Journal of Hepatology 1999;30:830–5. doi:10.1016/s0168-8278(99)80136-6
- 22Rudic JS, Poropat G, Krstic MN, et al. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews Published Online First: 12 December 2012. doi:10.1002/14651858.cd000551.pub3
- 23Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of Alkaline Phosphatase and Bilirubin Are Surrogate End Points of Outcomes of Patients With Primary Biliary Cirrhosis: An International Follow-up Study. Gastroenterology 2014;147:1338-1349.e5. doi:10.1053/j.gastro.2014.08.029
- 24Ursodeoxycholic acid 250mg capsules. Electronic Medicines Compendium. 2016.https://www.medicines.org.uk/emc/product/7253/smpc (accessed May 2023).
- 25Siegel JL, Jorgensen R, Angulo P, et al. Treatment With Ursodeoxycholic Acid Is Associated With Weight Gain in Patients With Primary Biliary Cirrhosis. Journal of Clinical Gastroenterology 2003;37:183–5. doi:10.1097/00004836-200308000-00018
- 26OCALIVA 10mg film-coated tablets. Electronic Medicines Compendium. 2018.https://www.medicines.org.uk/emc/product/7630/smpc (accessed May 2023).
- 27Nevens F, Andreone P, Mazzella G, et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med 2016;375:631–43. doi:10.1056/nejmoa1509840
- 28Obeticholic acid for treating primary biliary cholangitis. Technology appraisal guidance [TA443]. National Institute for Health and Care Excellence. 2017.https://www.nice.org.uk/guidance/ta443 (accessed May 2023).
- 29Improving Value in Specialised Services: Menu of Opportunities. NHS England. 2019.https://www.england.nhs.uk/wp-content/uploads/2019/04/improving-value-menu-opportunities-guide-v4.pdf (accessed May 2023).
- 30OCALIVA obeticholic acid dosing guide. Intercept Pharmaceuticals. 2020.https://ocalivahcp.com/wp-content/uploads/2020/04/ocaliva-obeticholic-acid-dosing-guide.pdf (accessed Mar 2021).
- 31Corpechot C, Chazouillères O, Rousseau A, et al. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med 2018;378:2171–81. doi:10.1056/nejmoa1714519
- 32Fibrazat XL 400mg Modified Release Tablets. Electronic Medicines Compendium. 2015.https://www.medicines.org.uk/emc/product/6547 (accessed May 2023).
- 33Fenofibrate 267mg Capsules. Electronic Medicines Compendium. 2018.https://www.medicines.org.uk/emc/product/4426/smpc (accessed May 2023).
- 34Barber K, Madden S, Allen J, et al. Elective Liver Transplant List Mortality: Development of a United Kingdom End-Stage Liver Disease Score. Transplantation 2011;92:469–76. doi:10.1097/tp.0b013e318225db4d
- 35Huet P-M, Deslauriers J, Tran A, et al. Impact of fatigue on the quality of life of patients with primary biliary cirrhosis. Am J Gastroenterology 2000;95:760–7. doi:10.1111/j.1572-0241.2000.01857.x
- 36Mells GF, Pells G, Newton JL, et al. Impact of primary biliary cirrhosis on perceived quality of life: The UK-PBC national study. Hepatology 2013;58:273–83. doi:10.1002/hep.26365
- 37Theal JJ, Toosi MN, Girlan L, et al. A randomized, controlled crossover trial of ondansetron in patients with primary biliary cirrhosis and fatigue. Hepatology 2005;41:1305–12. doi:10.1002/hep.20698
- 38Talwalkar JA, Donlinger JJ, Gossard AA, et al. Fluoxetine for the Treatment of Fatigue in Primary Biliary Cirrhosis: A Randomized, Double-Blind Controlled Trial. Dig Dis Sci 2006;51:1985–91. doi:10.1007/s10620-006-9397-5
- 39Silveira MG, Gossard AA, Stahler AC, et al. A Randomized, Placebo-Controlled Clinical Trial of Efficacy and Safety: Modafinil in the Treatment of Fatigue in Patients With Primary Biliary Cirrhosis. American Journal of Therapeutics 2017;24:e167–76. doi:10.1097/mjt.0000000000000387
- 40Primary Biliary Cholangitis Symptom Management. Patient Information Booklet. INTERCEPT PHARMA EUROPE LTD 2017.
- 41Pate J, Gutierrez JA, Frenette CT, et al. Practical strategies for pruritus management in the obeticholic acid-treated patient with PBC: proceedings from the 2018 expert panel. BMJ Open Gastroenterol 2019;6:e000256. doi:10.1136/bmjgast-2018-000256
- 42Questran Light 4g/sachet Powder for Oral Suspension. Electronic Medicines Compendium. 2021.https://www.medicines.org.uk/emc/product/10588/smpc (accessed May 2023).
- 43MedicinesComplete. MedicinesComplete. 2023.https://www.medicinescomplete.com/#/content/stockley/x00-2781 (accessed May 2023).
- 44Ghent CN, Carruthers SG. Treatment of pruritus in primary biliary cirrhosis with rifampin. Gastroenterology 1988;94:488–93. doi:10.1016/0016-5085(88)90442-8
- 45Bachs L, Elena M, Parés A, et al. COMPARISON OF RIFAMPICIN WITH PHENOBARBITONE FOR TREATMENT OF PRURITUS IN BILIARY CIRRHOSIS. The Lancet 1989;333:574–6. doi:10.1016/s0140-6736(89)91608-5
- 46Podesta A, Lopez P, Terg R, et al. Treatment of pruritus of primary biliary cirrhosis with rifampin. Digest Dis Sci 1991;36:216–20. doi:10.1007/bf01300759
- 47Bachs L, Parés A, Elena M, et al. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology 1992;102:2077–80. doi:10.1016/0016-5085(92)90335-v
- 48Rifadin 300mg Capsules. Electronic Medicines Compendium. 2019.https://www.medicines.org.uk/emc/product/6384/smpc (accessed May 2023).
- 49Bergasa NV, Talbot TL, Alling DW, et al. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology 1992;102:544–9. doi:10.1016/0016-5085(92)90102-5
- 50Joint Formulary Committee. British National Formulary. London: : BMJ Group, Pharmaceutical Press, and RCPCH Publications 2020. http://www.medicinescomplete.com (accessed May 2023).
- 51Rishe E, Azarm A, Bergasa N. Itch in Primary Biliary Cirrhosis: A Patients’ Perspective. Acta Derm Venereol 2008;88:34–7. doi:10.2340/00015555-0350
- 52Piriton Tablets. Electronic Medicines Compendium. 2021.https://www.medicines.org.uk/emc/product/20/smpc (accessed May 2023).
- 53Mayo MJ, Handem I, Saldana S, et al. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology 2007;45:666–74. doi:10.1002/hep.21553
- 54Sertraline 100mg Film-coated Tablets. Electronic Medicines Compendium. 2021.https://www.medicines.org.uk/emc/product/4356/smpc (accessed May 2023).
- 55Raszeja-Wyszomirska J, Miazgowski T. Osteoporosis in primary biliary cirrhosis of the liver. pg 2014;2:82–7. doi:10.5114/pg.2014.42502
- 56Guañabens N, Parés A, Ros I, et al. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. Journal of Hepatology 2005;42:573–7. doi:10.1016/j.jhep.2004.11.035
- 57FRAX Fracture Risk assessment Tool. University of Sheffield. 2008.https://www.sheffield.ac.uk/FRAX/tool.aspx?country=9 (accessed May 2023).
- 58Zein CO, Jorgensen RA, Clarke B, et al. Alendronate improves bone mineral density in primary biliary cirrhosis: A randomized placebo-controlled trial. Hepatology 2005;42:762–71. doi:10.1002/hep.20866