Shutterstock.com
After reading this article, you should be able to:
- Understand the prevalence and pathophysiology of pulmonary hypertension;
- Recognise typical presentation of a patient with pulmonary hypertension and diagnostic pathway;
- Identify targeted treatments used for pulmonary hypertension.
Introduction
Pulmonary hypertension (PH) comprises a heterogeneous number of haemodynamic conditions, all with a common feature of elevated resting pulmonary artery pressure. Joint guidelines from the European Society of Cardiology (ECS) and European Respiratory Society (ERS) define that a resting mean pulmonary artery pressure of ≥20mmHg is indicative of a diagnosis of PH[1]. The guidelines further categorise PH by underlying aetiology and can be seen in Figure 1.
PH is a rare disease, with current estimates suggesting a prevalence of around 1% worldwide. Owing to the presence of respiratory and cardiac causes of PH, prevalence is higher in patients aged over 65 years[2]. In the UK, there has been a 100% increase in prevalence within the past 10 years and it currently affects 125 people per million people[3]. The number of new referrals to PH specialist centres within the UK has also increased, from 1,861 in 2009/2010 to 3,585 in 2022/2023[3].
PH is a life-shortening, progressive disease that can manifest with debilitating symptoms, including breathlessness, fluid overload and arrhythmias. In general, symptoms of PH are usually dictated by the right ventricle dysfunction[2,4].
Prior to targeted vasodilator therapy, supportive therapy was only available for symptom control, such as diuretics for fluid overload and oxygen to treat hypoxemia. In 1995, IV epoprostenol was approved, paving the way for almost three decades of new targeted therapies, aimed to improve exercise capacity, improve quality of life and improve mortality and morbidity. While only epoprostenol has demonstrated a definitive mortality benefit, all agents have shown improvement in quality of life and exercise capacity[4].
This article will outline the signs and symptoms of PH and provide an overview of how the condition is diagnosed and managed, including how pharmacists can contribute to patient care.
Signs and symptoms
PH commonly presents with progressive breathlessness. As right ventricular dysfunction develops, patients can experience syncope, peripheral oedema and, ultimately, arrhythmias[4]. Angina chest pain may occur owing to exertional cardiac hypoperfusion in the setting of right heart failure. As the disease progresses, patients may present with tachyarrhythmias, most commonly atrial flutter, which can cause sudden decompensation and death[5].
Many of the symptoms are non-specific to PH, such as fatigue, fluid retention and syncope; therefore, the disease may be mistaken for other, more common pathologies, particularly cardiac and respiratory, including asthma, chronic obstructive pulmonary disease (COPD) and left ventricular systolic dysfunction or congestive heart disease. This can result in delayed presentation, leading to more severe disease at the point of diagnosis and poorer patient outcomes. The median duration between symptom onset and diagnosis is around 2.5 years[6].
Clinical examination in the early stage may be unremarkable or may reveal a loud second heart sound or pan-systolic murmur. A raised jugular venous pressure (JVP) and pedal oedema can indicate signs of right heart failure or overload. Other symptoms may be associated with later stages of the disease or more severe disease. These can include:
- Exertional chest pain, owing to dynamic compression of coronary arteries;
- Hoarseness, owing to compression of laryngeal nerve;
- Wheeze, cough or lower respiratory tract infection, owing to bronchi compression[1].
It is also prudent to consider progression of underlying causes of PH, such as left ventricular systolic dysfunction, progression in lung disease such as fibrosis, COPD, emphysema or connective-tissue diseases, such as scleroderma and lupus (see Figure 2)[1]. Right ventricular (RV) failure can be classed as either forward or backwards[1]. Forward failure is associated with RV failure to generate adequate cardiac output, leading to cardiogenic shock. Backward failure is attributed to RV failure to decongest the systemic venous system, leading to high central venous pressures with systematic congestion[1].
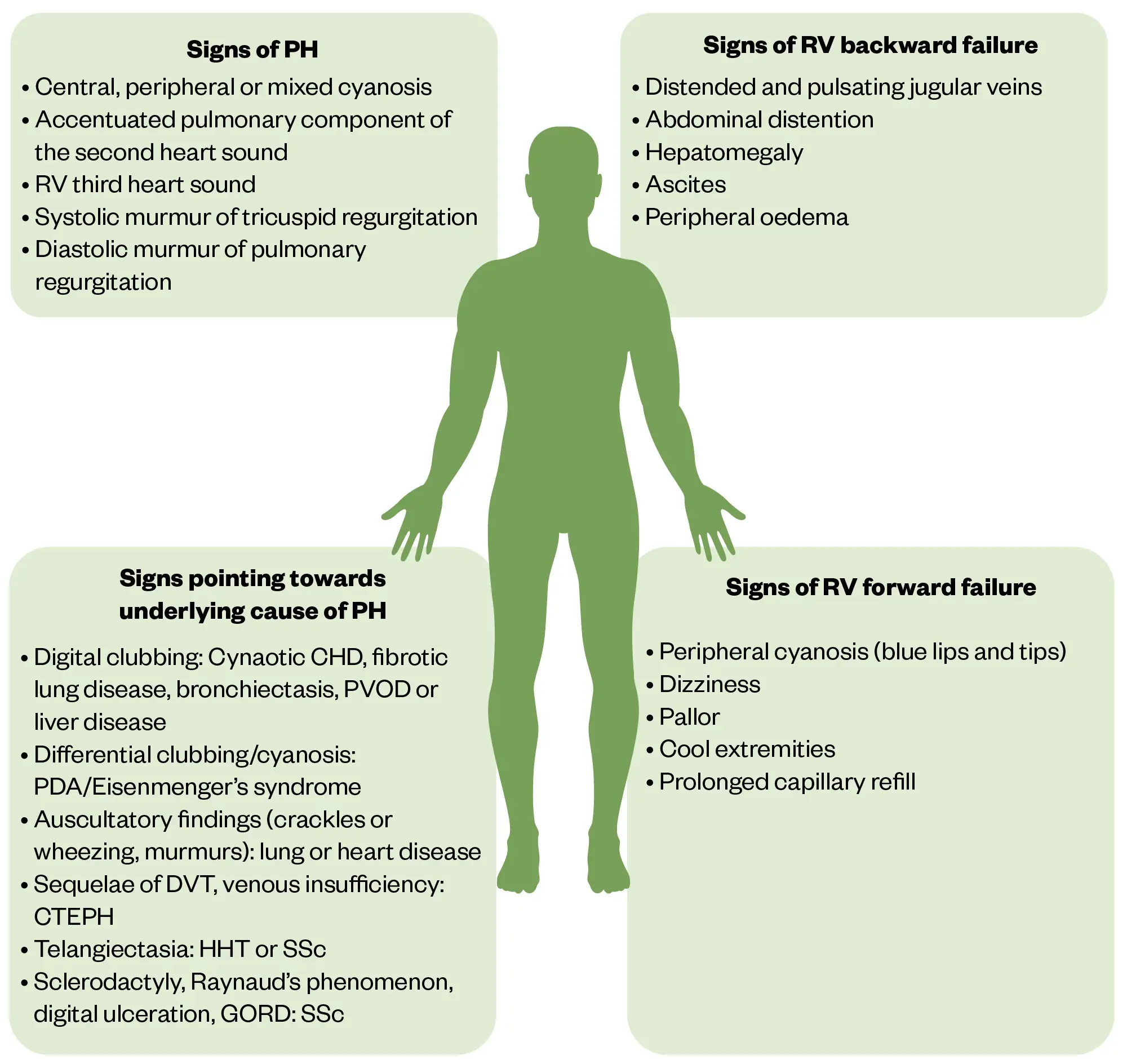
Reproduced with permission from The European Society of Cardiology & European Respiratory Society
Overview of pathophysiology
The pathophysiology of PH can vary depending on the underlying aetiology. This section will focus primarily on Group I PH, known as pulmonary arterial hypertension (PAH). PAH is further categorised according to its aetiology, with idiopathic PAH comprising the majority of cases, followed by PAH associated with connective tissue diseases and congenital heart disorders[7]. It is commonly characterised by angioproliferative vasculopathy in the pulmonary arterioles[8,9]. This affects pre-capillary arterioles by smooth muscle proliferation, dysfunction, inflammation and thrombosis, ultimately reducing lumen diameter, giving a disordered appearance. This in turn increases right ventricle afterload, with the resulting right heart failure being the most common cause of death[10].
The pathogenesis of PAH is most likely multi-factorial, involving multiple pathways rather than a single mechanism. In all cases there is an imbalance of vasoconstricting and vasodilating substances in the pulmonary arteries. Increases in vasoconstricting substances (such as thromboxane, endothelin and serotonin) and reduced levels of vasodilatory substances (such as nitric oxide) are commonly observed in PAH patients[11]. Vascular proliferation has also been implicated in PAH patients. Inappropriate activation of transcription factors can promote smooth muscle propagation[10].
Risk factors
Historically, PAH was thought to affect younger individuals, mostly females[12,13]. While this is certainly the case for heritable PH, which affects twice as many females than males, recent data from the UK-wide PH audit suggest PAH is now diagnosed as often in older patients, with an equal distribution between sexes[12]. There are several genetic mutations associated with PAH, more commonly these include BMPR2, ACVRL1 and CAV1; BMPR2 mutations have been identified in 80% of families with PAH[14,15]. Other risk factors include connective tissue diseases, which are the second leading cause of PAH after idiopathic form. Systemic sclerosis is the most common precipitating disease for connective tissue disorder PAH, and represents a severe complication occurring in 8–12% of patients with systemic sclerosis[16].
Thromboembolism is a further risk factor for PH, with an estimated incidence of progression from acute clot to chronic thromboembolic pulmonary hypertension occurring in approximately 0.1–11.8% of patients[17–19]. There are several drugs and toxins associated with the development of PH, which include illicit substances (such as methamphetamines and cocaine) or prescribed medicines (such as anti-cancer drugs dasatinib, interferon alpha or mitomycin)[20].
Diagnosis
Often patients can present in right-sided heart failure, with no obvious other cardiac or respiratory causes. With increased awareness of the disease and increased visibility of specialist centres, it may aid earlier diagnosis and treatment initiation, resulting in improved patient prognosis and outcomes[8,13].
As per the most recent 2022 ERS/ECS guidelines, diagnosis is multimodal[1]. A full patient history and examination should be taken to understand duration of symptoms and exclude other potential causes for ongoing breathlessness. This should include onset of breathlessness, presence of peripheral oedema and signs of RV failure (as listed above) as well as other symptoms that may indicate an underlying cause for PH, such as nocturnal waking/cough indicative of asthma, recurrent chest infections indicative of COPD or skin tightening and hair loss associated with connective tissue diseases[6,11,16].
Electrophysiology (ECG), chest radiography (X-ray) and pulmonary function tests (PFTs) may identify an alternate cause of symptoms. They are also helpful in excluding or identifying underlying aetiologies or disease. Chest radiography can be normal; however, an enlarged cardiac silhouette and pulmonary arteries may also be found. Additionally, signs of underlying causes of PH, such as left heart disease or lung disease, may be identified on X-ray. PFTs and analysis of arterial blood gas (ABG) are necessary to distinguish between PH groups, and assess comorbidities and the need for supplemental oxygen. Often PFTs may be normal, but can demonstrate mild restrictive, obstructive or combined abnormalities[21,22].
Echocardiography can provide comprehensive information on the right and left heart morphology and give estimates on haemodynamic parameters, including mean pulmonary artery pressure. Echocardiography alone is insufficient to confirm a diagnosis of PH, although it can be useful to determine an underlying cause; for example, in Group 2 PH, where patients may demonstrate left systolic dysfunction with preserved or reduced ejection fraction[23].
Other modalities may be used, such as computer-tomography (CT), cardiac magnetic resonance imaging (MRI) and ventilation/perfusion (V/Q) scans, alongside cardiopulmonary exercise testing, which can all contribute to the diagnostic process and uncovering the cause of PH. Blood tests and immunology can also demonstrate underlying comorbidities and possible causes or complications of PH; for example, clotting disorders, such as antiphospholipid syndrome or thrombophilia, or presence of connective tissue disorders with positive immunology[1].
Right heart catheterisation (RHC) is the gold-standard technique for diagnosing and classifying PH. This is an invasive procedure where a Swan-Ganz catheter is inserted under local anaesthetic. This is commonly undertaken within a cath lab, under fluoroscopy guidance. When performed in PH centres, the frequencies of serious adverse events and procedure-related mortality are low (1.1% and 0.055%, respectively)[24]. Further measurements can be taken, including right atrial pressure, pulmonary artery wedge pressure, vascular resistance and cardiac output.
Management of pulmonary hypertension
Management of PH can depend on a multitude of factors, including the type (or group), severity and patient functional class, alongside other comorbidities or interacting agents.
Management can be divided into three distinct pathways: targeted therapy, supportive therapies and surgical interventions. Historically, without targeted therapy, prognosis for patients was poor, with evidence suggesting a maximum survival of 18 months to 3 years from the point of diagnosis[25]. While PH remains a life-shortening illness, with aggressive treatment and initiation of targeted therapies, prognosis (depending on PH type and severity) has been quoted as ranging from 3–5 years[25,26].
Supportive measures include oxygen, diuretics and analgesia. While these agents still play a role in managing symptoms, they are often used in conjunction with targeted therapies[1]. Diuretics should be used to manage fluid balance, especially in the presence of right heart failure or overload. Patients may require higher than usual doses to maintain a euvolemic state. Anticoagulants should be used in line with current licences; patients with chronic thromboembolic pulmonary hypertension will typically require life-long anticoagulation[1].
Targeted agents act by reducing pulmonary artery pressure. The mechanism of all targeted therapies is by one of three pathways, which either inhibit vasoconstriction or enable/promote vasodilatation (see Figure 3)[1].
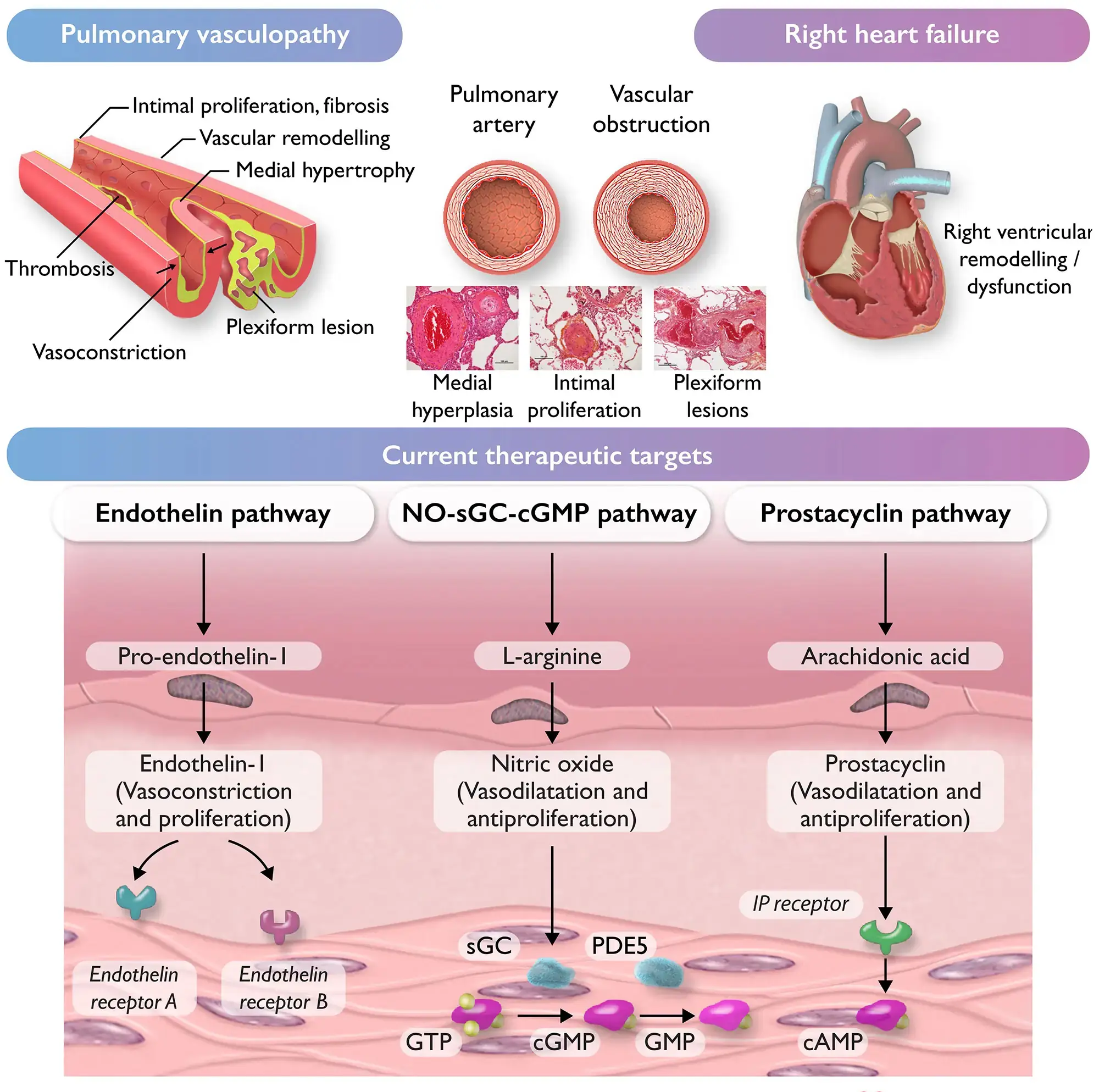
Reproduced with permission from The European Society of Cardiology & European Respiratory Society
Many targeted therapies are specialist medicines, meaning that these are typically managed and prescribed by specialist centres and delivered by homecare providers. There may be lack of awareness or knowledge of these drugs within primary care and they may not be clearly identifiable or included in primary care records or electronic care summaries.
There is a high degree of research within PH, in combination with the relatively small cohort of patients, meaning it may be common for patients to be enrolled in clinical trials. This can further complicate their management if enrolled in blinded trials. Pharmacy teams should ensure that these medicines are added to records for information only, and that patients should be encouraged to always take these medicines as directed and to not stop unless specifically advised to by their PH specialist centre. Once started, these medicines will be lifelong, but dosing schedules may change and it is imperative that records are kept up to date with clinic letters. Patients may be the best source of information for teams to confirm with if in doubt.
Phosphodiesterase 5 (PDE-5)
Currently, phosphodiesterase 5 (PDE-5) inhibitors sildenafil and tadalafil are both licensed for use in PH. Used regularly and typically lifelong, doses can range from 25–100mg three times per day and 20–40mg once daily for sildenafil and tadalafil, respectively[1]. These agents work by inhibiting phosphodiesterase and, as a result, increase intracellular concentrations of cyclic guanosine monophosphate (GMP), resulting in smooth muscle relaxation and reduction in cellular proliferation. Both agents have been demonstrated to improve exercise and functional capacity[27,28].
PDE-5 inhibitors are contraindicated with other nitrates (or drugs working within a similar mechanism) that are commonly prescribed in angina or ischaemic heart disease, including isosorbide mono/dinitrate and nicorandil[29]. There must be an appropriate wash-out period prior to treatment initiation with PDE-5 inhibitors, which will typically be conducted by specialist PH centres. Concurrent use with nitrates (including when required glyceryl trinitrate sublingual sprays/tablets) can result in a sudden drop in systolic blood pressure that can be fatal[29]. While generally well tolerated, there have been reports of nonarteritic anterior ischaemic optic neuropathy, which can be irreversible, alongside sudden hearing loss[30,31]. Presentation of either visual or hearing disturbance should prompt immediate review and potential discontinuation of the agent.
Endothelin receptor antagonists
Endothelin is a potent vasoconstrictor of vascular smooth muscle. Three endothelin receptor antagonists (ERAs) are currently licensed for use in PH: ambrisentan, bosentan and macitentan. All three agents have demonstrated improved exercise capacity and time to clinical worsening[32–34]. Dose contraindications and safety profiles can be seen in the table below[35–38]. ERAs are contraindicated in pregnancy owing to teratogenic effects and should only be used in patients of childbearing age who are using two forms of contraception (such as hormonal contraception and a barrier method)[39].
Prostanoids
Prostacyclin is a potent vasodilator with antiproliferative effects. Mainly used in parenteral form, epoprostenol is infused continuously through an indwelling central line. It is commonly started at a dose of 2ng/kg/min and slowly titrated up to a target of 20–24ng/kg/min (centre specific)[25,40]. It was the first PH drug to demonstrate efficacy in exercise capacity, haemodynamics and, unlike other targeted agents, survival[41]. It has a half-life of less than ten minutes, resulting in the need for continuous infusion. This agent can be poorly tolerated and associated with persistent side effects, including diarrhoea, myalgia, and nausea and vomiting. There is the added risk of complications associated with vascular access. This option is only used in patients who have severe PH or who demonstrate more severe symptoms or greater limitations on daily life (known as functional class).
In a randomised controlled trial, nebulised iloprost was found to improve exercise capacity and reduce pulmonary artery pressures; however, it has to be inhaled seven to nine times per day[42]. Compliance can commonly be an issue with inhaled iloprost, along with the dexterity required to use and wash the device. Treprostinil can be continually infused both intravenously and subcutaneously. Subcutaneous administration can enable alternative formulation for patients with severe disease but complex or limited vascular access options. Subcutaneous treprostinil has been demonstrated to increase exercise capacity, but is associated with site pain and inflammation, which can be challenging to manage[43,44].
Selexipag is the first oral non-prostanoid selective prostacyclin receptor agonist. It is licensed for use in PH and has demonstrated benefit in doses ranging from 200 micrograms twice daily to 1,600 micrograms twice daily[45]. Adverse effect burden can be high, with many patients complaining of headache, gastrointestinal upset, jaw pain and nausea. It can be a useful third agent when patients are unable to tolerate or manage intravenous/subcutaneous prostanoids[25,40,41].
Supportive medicines can be utilised to help alleviate side effect; these may include antiemetics (ondansetron) and antidiarrheals (loperamide) alongside simple analgesia.
Non-pharmaceutical interventions
Lung transplantation can offer a cure for certain patients, but this is often not a viable option given the common delayed presentation and comorbidities within patient cohorts. Lung transplantation is only available in six specialist centres within the UK, with a total of 106 transplants performed in 2021/2022[46]. Patients with PH can face several challenges in the form of higher perioperative and intraoperative risks, inequities in allocation and less favourable long-term outcomes.
Further surgical intervention can be offered to patients, including pulmonary endarterectomy (PEA), and balloon pulmonary angiography (BPA) may be offered to patients with Group IV disease (chronic thromboembolic disease), which can be curative in certain patients and in others allow for drastic improvement in symptom burden[47].
Self-care
PH is a progressive and, for many individuals, incurable disease. Pharmacotherapy can delay progression and improve exercise capacity. Patients are recommended to keep active, lead a healthy lifestyle and try to maintain independence and avoid deconditioning. Continued treatment for other underlying causes (e.g. left heart dysfunction or lung diseases) should continue and be maximised to avoid further progression of pulmonary hypertension.
Best practice
- Patients should be supported and managed in a specialist centre with dedicated pharmacy resource to care for patients with PH;
- Targeted therapy involves specialist medicines, which may not be clear on typical admission documents and can be easily missed on medicines history or reconciliation;
- Missed doses of targeted therapies can result in dangerous rebound pulmonary hypertension that is associated with poorer prognosis and clinical deterioration;
- Patients may be an “expert” in their treatment; therefore, they may be the best source for information on medicines, dosing or infusion rates;
- Patients may be comorbid and require intensive management of medicines to optimise outcomes;
- PH is a rare disease with an ongoing research focus where patients may be enrolled in trials, resulting in uncertainty over investigational agents that may require unblinding.
- 1Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal. 2022;43:3618–731. https://doi.org/10.1093/eurheartj/ehac237
- 2Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. The Lancet Respiratory Medicine. 2016;4:306–22. https://doi.org/10.1016/s2213-2600(15)00543-3
- 3National Audit of Pulmonary Hypertenion 14th Annual Report. NHS Digital. 2022. https://digital.nhs.uk/data-and-information/publications/statistical/national-pulmonary-hypertension-audit/14th-annual-report (accessed March 2024)
- 4Kiely DG, Elliot CA, Sabroe I, et al. Pulmonary hypertension: diagnosis and management. BMJ. 2013;346:f2028–f2028. https://doi.org/10.1136/bmj.f2028
- 5Bandorski D, Heibel S, Höltgen R, et al. Incidence and prognostic significance of malignant arrhythmias during (repetitive) Holter electrocardiograms in patients with pulmonary hypertension. Front. Cardiovasc. Med. 2023;10. https://doi.org/10.3389/fcvm.2023.1084051
- 6Maron BA, Abman SH, Elliott CG, et al. Pulmonary Arterial Hypertension: Diagnosis, Treatment, and Novel Advances. Am J Respir Crit Care Med. 2021;203:1472–87. https://doi.org/10.1164/rccm.202012-4317so
- 7Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary Arterial Hypertension. Chest. 2010;137:376–87. https://doi.org/10.1378/chest.09-1140
- 8Lan N, Massam B, Kulkarni S, et al. Pulmonary Arterial Hypertension: Pathophysiology and Treatment. Diseases. 2018;6:38. https://doi.org/10.3390/diseases6020038
- 9Prins KW, Thenappan T. World Health Organization Group I Pulmonary Hypertension. Cardiology Clinics. 2016;34:363–74. https://doi.org/10.1016/j.ccl.2016.04.001
- 10Tuder RM, Archer SL, Dorfmüller P, et al. Relevant Issues in the Pathology and Pathobiology of Pulmonary Hypertension. Journal of the American College of Cardiology. 2013;62:D4–12. https://doi.org/10.1016/j.jacc.2013.10.025
- 11Farber HW, Loscalzo J. Pulmonary Arterial Hypertension. N Engl J Med. 2004;351:1655–65. https://doi.org/10.1056/nejmra035488
- 12Lau EMT, Giannoulatou E, Celermajer DS, et al. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol. 2017;14:603–14. https://doi.org/10.1038/nrcardio.2017.84
- 13Montani D, Girerd B, Jaïs X, et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J. 2020;2004229. https://doi.org/10.1183/13993003.04229-2020
- 14Morrell NW, Aldred MA, Chung WK, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801899. https://doi.org/10.1183/13993003.01899-2018
- 15Evans JDW, Girerd B, Montani D, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. The Lancet Respiratory Medicine. 2016;4:129–37. https://doi.org/10.1016/s2213-2600(15)00544-5
- 16Thoreau B, Mouthon L. Pulmonary arterial hypertension associated with connective tissue diseases (CTD-PAH): Recent and advanced data. Autoimmunity Reviews. 2024;23:103506. https://doi.org/10.1016/j.autrev.2023.103506
- 17Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Haemost. 2014;112:598–605. https://doi.org/10.1160/th13-07-0538
- 18Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49:1601792. https://doi.org/10.1183/13993003.01792-2016
- 19Golpe R, Pérez-de-Llano LA, Castro-Añón O, et al. Right ventricle dysfunction and pulmonary hypertension in hemodynamically stable pulmonary embolism. Respiratory Medicine. 2010;104:1370–6. https://doi.org/10.1016/j.rmed.2010.03.031
- 20Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. https://doi.org/10.1183/13993003.01913-2018
- 21Remy-Jardin M, Ryerson CJ, Schiebler ML, et al. Imaging of pulmonary hypertension in adults: a position paper from the Fleischner Society. Eur Respir J. 2021;57:2004455. https://doi.org/10.1183/13993003.04455-2020
- 22Sun X-G, Hansen JE, Oudiz RJ, et al. Pulmonary function in primary pulmonary hypertension. Journal of the American College of Cardiology. 2003;41:1028–35. https://doi.org/10.1016/s0735-1097(02)02964-9
- 23Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2010;23:685–713. https://doi.org/10.1016/j.echo.2010.05.010
- 24Hoeper MM, Lee SH, Voswinckel R, et al. Complications of Right Heart Catheterization Procedures in Patients With Pulmonary Hypertension in Experienced Centers. Journal of the American College of Cardiology. 2006;48:2546–52. https://doi.org/10.1016/j.jacc.2006.07.061
- 25McLaughlin VV, Shillington A, Rich S. Survival in Primary Pulmonary Hypertension. Circulation. 2002;106:1477–82. https://doi.org/10.1161/01.cir.0000029100.82385.58
- 26Chang KY, Duval S, Badesch DB, et al. Mortality in Pulmonary Arterial Hypertension in the Modern Era: Early Insights From the Pulmonary Hypertension Association Registry. JAHA. 2022;11. https://doi.org/10.1161/jaha.121.024969
- 27Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil Citrate Therapy for Pulmonary Arterial Hypertension. N Engl J Med. 2005;353:2148–57. https://doi.org/10.1056/nejmoa050010
- 28Barnes H, Brown Z, Burns A, et al. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database of Systematic Reviews. 2019;2019. https://doi.org/10.1002/14651858.cd012621.pub2
- 29Schwartz BG, Kloner RA. Drug Interactions With Phosphodiesterase-5 Inhibitors Used for the Treatment of Erectile Dysfunction or Pulmonary Hypertension. Circulation. 2010;122:88–95. https://doi.org/10.1161/circulationaha.110.944603
- 30GILL E, AZULAY H. MORE THAN MEETS THE EYE: PDE5i AND VISION CHANGES. CHEST. 2023;164:A6036. https://doi.org/10.1016/j.chest.2023.07.3891
- 31Barreto MA de SC, Bahmad F Jr. Phosphodiesterase Type 5 Inhibitors and sudden sensorineural hearing loss. Brazilian Journal of Otorhinolaryngology. 2013;79:727–33. https://doi.org/10.5935/1808-8694.20130133
- 32Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan Therapy for Pulmonary Arterial Hypertension. N Engl J Med. 2002;346:896–903. https://doi.org/10.1056/nejmoa012212
- 33Badesch DB, Feldman J, Keogh A, et al. ARIES‐3: Ambrisentan Therapy in a Diverse Population of Patients with Pulmonary Hypertension. Cardiovascular Therapeutics. 2011;30:93–9. https://doi.org/10.1111/j.1755-5922.2011.00279.x
- 34Said K. Macitentan in pulmonary arterial hypertension: The SERAPHIN trial. Global Cardiology Science and Practice. 2014;2014:20. https://doi.org/10.5339/gcsp.2014.20
- 35Volibris 5 mg film coated tablets. Electronic Medicines Compendium. 2022. https://www.medicines.org.uk/emc/product/6258/smpc#gref (accessed March 2024)
- 36British National Formulary. Medicines Complete. 2024. https://www.medicinescomplete.com/#/browse/bnf (accessed March 2024)
- 37Opsumit 10mg film coated tablets. Electronic Medicines Compendium. 2023. https://www.medicines.org.uk/emc/product/5223/smpc#gref (accessed March 2024)
- 38Bosentan 125mg film-coated tablets. Electronic Medicines Compendium. 2021. https://www.medicines.org.uk/emc/product/821/smpc#gref (accessed March 2024)
- 39Lüscher TF, Barton M. Endothelins and Endothelin Receptor Antagonists. Circulation. 2000;102:2434–40. https://doi.org/10.1161/01.cir.102.19.2434
- 40Mohammadi A, Matos WF, Intriago C, et al. Use of Epoprostenol in the Treatment of Pulmonary Arterial Hypertension. Cureus. 2021. https://doi.org/10.7759/cureus.18191
- 41Barst RJ, Rubin LJ, Long WA, et al. A Comparison of Continuous Intravenous Epoprostenol (Prostacyclin) with Conventional Therapy for Primary Pulmonary Hypertension. N Engl J Med. 1996;334:296–301. https://doi.org/10.1056/nejm199602013340504
- 42Olschewski H, Simonneau G, Galiè N, et al. Inhaled Iloprost for Severe Pulmonary Hypertension. N Engl J Med. 2002;347:322–9. https://doi.org/10.1056/nejmoa020204
- 43SIMONNEAU G, BARST RJ, GALIE N, et al. Continuous Subcutaneous Infusion of Treprostinil, a Prostacyclin Analogue, in Patients with Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2002;165:800–4. https://doi.org/10.1164/ajrccm.165.6.2106079
- 44White RJ, Levin Y, Wessman K, et al. Subcutaneous Treprostinil is Well Tolerated with Infrequent Site Changes and Analgesics. Pulm. circ. 2013;3:611–21. https://doi.org/10.1086/674304
- 45Bruderer S, Hurst N, Remenova T, et al. Clinical pharmacology, efficacy, and safety of selexipag for the treatment of pulmonary arterial hypertension. Expert Opinion on Drug Safety. 2017;16:743–51. https://doi.org/10.1080/14740338.2017.1328052
- 46Annual Report of Cardiothoracic Organ Transplantation. NHS England. 2022. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/27816/nhsbt-annual-report-on-cardiothoracic-organ-transplantation-202122.pdf (accessed March 2024)
- 47Shimahara Y, Suzuki S, Fujiyoshi T, et al. Balloon pulmonary angioplasty followed by pulmonary endarterectomy: Combination treatment for high-surgical-risk patients with chronic thromboembolic pulmonary hypertension. Interdisciplinary CardioVascular and Thoracic Surgery. 2023;36. https://doi.org/10.1093/icvts/ivad031