This content was published in 2004. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
As renal function declines, a variety of metabolic disturbances manifest themselves. Some patients will have altered biochemistry when their renal impairment is fairly mild, but by the time renal failure has become severe (a glomerular filtration rate or creatinine clearance below 10ml/minute), most patients have abnormal serum calciumand phosphate levels (see Panel 1 for normal reference ranges), falling vitamin D levels, and rising levels of parathyroid hormone (PTH). These result in a variety of clinical symptoms.
Attempts to correct such abnormalities include the use of vitamin D and calcium-based phosphate binders. Treatment is usually successful initially, but there are worries that the therapeutic use of these products can contribute to cardiovascular and soft-tissue calcification in the long term.
Calcium homeostasis
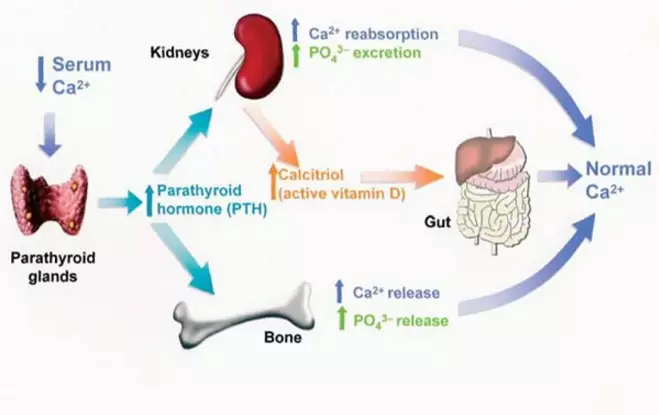
The interaction between serum calcium,active vitamin D, phosphate, and PTH in thehealthy individual is shown in Figure 1.
Most calcium in the body is stored as calcium phosphate in the bones, which are in a constant state of breakdown and repair, and only a small amount circulates in the blood. Relatively small amounts are absorbed from dietary sources (eg, dairy products) and excreted in the urine to maintain overall calcium balance.
Too low a level, hypocalcaemia, can produce tetany, convulsions and cardiac arrythmias, which can require the urgent administration of intravenous calcium gluconate.
Hypercalcaemia most commonly occurs as a complication of bone breakdown in metastatic malignancies or prolonged immobility, or as a consequence of excessive vitamin D intake. Symptoms include muscle weakness, nausea, thirst, confusion and constipation.
Parathyroid hormone
Serum calcium levels are tightly controlled by PTH, a small protein (84 amino acids) secreted from the parathyroid glands in the neck in response to any fall in serum calcium. PTH restores serum calcium in three ways:
- It increases bone breakdown (osteoclast-mediated) to liberate free calcium (and phosphate)
- It increases vitamin D activation in the kidney, which in turn increases absorption of calcium from the gastrointestinal tract
- It increases reabsorption of calcium in the renal tubules to reduce renal losses, although phosphate excretion is promoted
Primary hyperparathyroidism is most commonly caused by tumours and manifests with hypercalcaemia and osteoporosis. In chronic kidney disease (CKD) secondary hyperparathyroidism often develops.
Panel 1: Typical reference ranges
Serum calcium (adjusted) 2.2–2.6mmol/L
Serum phosphate 0.7–1.4mmol/L
These are typical references ranges for healthy adults. Slightly different targets are set for patients with renal failure in the professional guidelines [1,2]
Identify knowledge gaps
1. How do problems with calcium, phosphate and parathyroid hormone arise in chronic kidney disease?
2. How can these be treated or prevented?
The Royal Pharmaceutical Society’s areas of competence for pharmacists are listed in “Plan and record”, (available at: www.rpsgb.org/education). This article relates to “drug therapy” (see appendix 4 of “Plan and record”).
3. What new therapies are on the horizon? Before reading on, think about how this article may help you to do your job better.
Vitamin D
Cholecalciferol (vitamin D3) is made in the skin when exposed to adequate sunlight but many people also need dietary sources, which supply ergocalciferol (vitaminD2). Cholecalciferol and ergocalciferol, whether provided naturally or by pharmaco-logical supplementation, are inactive and must be converted to an active form to be useful.
Hydroxylation of cholecalciferol at the 1α position is carried out in the kidney to produce 1α-hydroxycholecalciferol (alfacalcidol) and then at the 25-position in the liver to form the fully active 1,25-dihydroxycholecal-ciferol (calcitriol), as shown in Figure 2.
In CKD, renal hydroxylation of vitamin D precursors is impaired and this means calcium absorption from the gut falls, leading to a reduction in circulating serum calcium, and eventually symptomatic hypocalcaemia.
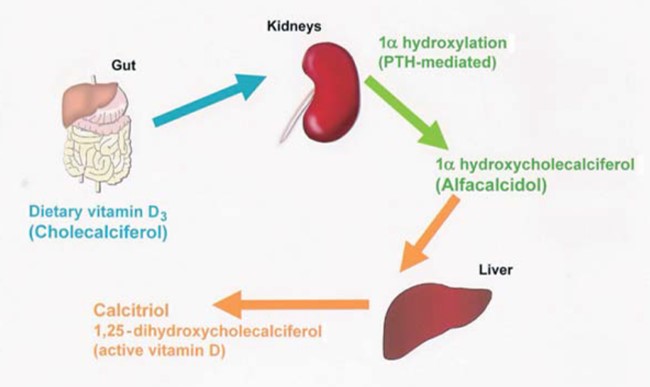
Calcium sensing receptors on the parathyroid glands detect this fall in calcium and increase production (by reducing negative feedback) of PTH to compensate. Due to low calcitriol levels, however, hypocalcaemia cannot be fully compensated, and PTH levels rise still further, resulting in secondary hyperparathyroidism.
Consequences of secondary hyperparathyroidism include renal bone disease (see later), a reduced response to epoetin and cardiomyopathy. However, cardiovascular calcification is by far the most serious development. Cardiovascular calcification may contribute significantly to the cardiovascular mortality of renal patients, many of whom also have co-morbidities, such as diabetes mellitus, dyslipidaemia, cardiac hypertrophy and hypertension. It is now well known that raised PTH and “calcium-phosphate product” (obtained by multiplying the serum levels of calcium and phosphate) are associated with increased mortality in patients on dialysis in retrospective studies. Calcification can involve the myocardium itself or its valves and the coronary arteries, but is also seen in organs such as the lungs, kidneys, joints and eyes.
Over time, chronic stimulation of the parathyroid gland causes parathyroid hyperplasia. Negative feedback control by serum calcium is diminished due to reduced expression of both vitamin D receptors and calcium sensing receptors in the hyperplastic tissue. As a result, ever higher concentrations of circulating calcium are required to suppress PTH release. High PTH begins to mobilise calcium from sources in the bones, causing bone problems (see later) additional to those caused by hypocalcaemia. If PTH levels remain high the hyperplasia becomes nodular, PTH secretion becomes uncontrolled and the patient is said to have developed tertiary hyperparathyroidism. Early suppression of PTH is important, because uncontrolled tertiary hyperparathyroidism requires surgical para-thyroidectomy.
Phosphate levels in renal failure
If kidney function decreases, phosphate excretion decreases. Phosphate retention is also implicated in driving secondary hyperparathyroidism — at high blood concentrations phos
phate can stimulate the parathyroid gland directly. Phosphate accumulation is accompanied by further falling calcium levels, aggravating both hypocalcaemia and secondary hyperparathyroidism. Hyperphosphataemia aggravates the itching, nausea, and erythropoietin resistance that is caused by the uraemia of chronic renal impairment.
Renal bone disease
Hypocalcaemia and secondary hyperparathyroidism are responsible for a variety of bone problems and increased fracture risk. Patients with CKD can complain of bone and joint pains, which tend to be vague and are commonly located in the lower back, hips, knees and legs. More severe pain may be a sign of fractures or collapsed vertebrae.
Renal bone disease can be broadly classified into “high turnover” and “low turnover” (see Panel 2). High-turnover disease is characterised by an excess of PTH and is commonly seen in late renal disease. Increased osteoclast activity leads to bone breakdown and fibrosis. There is haphazard deposition of bone and metastatic calcification is often present.
Treatment
The Department of Health website contains medicines management guidelines for renal patients [3]. This offers basic information on controlling calcium and phosphate as part of a general review and is suitable for practitioners inexperienced in the care of CKD. However, clearer targets have been defined, at least for dialysis patients, in the UK Renal Association Standards [1] and more recently, the US Kidney Disease Outcomes Quality Initiative (K/DOQI) [2]. The US guidelines are both stricter and more complicated, additionally setting limits for calcium-phosphate product. It also outlines suitable standards for earlier, less severe renal disease. Furthermore, they offer fairly prescriptive options for treatment of a variety of combinations of blood results and symptoms. Until updated UK guidelines become available, prescribers are faced with slightly conflicting advice, all based on the latest observational and trial data at the time of preparation. Targets derived from observational data showing an increased mortality in patients with uncontrolled electrolytes may simply be reflecting other, un-controlled variables. In addition, in all healthcare systems only a minority of patients on dialysis currently achieve all the targets set out in any guidelines for these parameters.
Activated vitamin D sterols
Neither alfacalcidol nor calcitriol require hydroxylation in the kidney and they are considered as “activated vitamin D” for renal patients.
Vitamin D sterols are effective at correcting hypocalcaemia (as described earlier, vitamin D increases gut absorption of calcium) and suppressing the parathyroid gland directly (via vitamin D receptors on the gland surface). This inhibits PTH release in a similar manner to the calcium sensing receptor and so tackles hyperparathyroidism.
Therapy is initiated at 0.25μg daily and titrated upwards. These two mechanisms lead to a reduction in PTH secretion. Unfortunately, when a sufficient dose of vitamin D is used to suppress PTH an unwanted excessive rise in increased serum calcium can result. This aggravates the elevated calcium-phosphate product and can result in calcium phosphate deposits in soft tissues. There has been a long and rather unproductive search for a vitamin D sterol that suppresses PTH without increasing calcium absorption in the gastrointestinal tract.
Pulsing the vitamin D as two or three doses each week seems to suppress PTH with a reduced risk of hypercalcaemia.
Panel 2: Treatment-related conditions
Low-turnover bone disease Low-turnover bone disease is commonly due to the oversuppression of PTH with vitamin D sterols and calcium-based phosphate binders.
Low-turnover disease is characterised by low PTH levels leading to reduced bone mineralisation and resorption, especially since slightly higher levels of PTH seem to be needed to maintain normal bone turnover in CKD. Patients with diabetes and those on continuous ambulatory peritoneal dialysis appear to be more susceptible to this type of bone disease. In addition, vitamin D deficiency can lead to osteomalacia.
Another cause of low-turnover disease is aluminium toxicity from binders or, formerly (before the development of adequate purification systems), long-term exposure to aluminium in the tap water used to maintain haemodialysis.
Calcium phosphate deposition Serum calcium rises following administration of vitamin D and calcium-based phosphate binders. However, the administration of these treatments can result in the deposition of calcium phosphate crystals in organs. Calcium phosphate deposition in the small blood vessels in skin can lead to intense itching and, in severe cases, acute inflammation, calcification and necrosis of the skin, termed calciphylaxis. Although rare, calciphylaxis has a mortality rate of up to 80 per cent, due to the development of sepsis.
Phosphate binders
Dietary restriction should be the first means of controlling serum phosphate and all patients with kidney disease should receive advice from qualified dietitian sat an early stage of the disease. Unfortunately, many renal patients are so anorexic or mal-nourished that phosphate-containing foods, such as red meat and dairy products, cannot be cut out without compromising the supply of other nutrients. Similarly, haemodialysis removes phosphate but high volumes of distribution in the body mean that serum phosphate levels soon rebound after the session ends.
If dietary restriction fails to control serum phosphate, both dialysing and non-dialysing patients will require phosphate binders. These are compounds that bind dietary phosphate in the gastrointestinal tract to prevent absorption into the body. Salts of calcium and aluminium, which lead to the production of insoluble phosphates in the gut, are the traditional choices. Magnesium salts are rarely used now because of the risk of hypermagnesaemia in renal failure. Aluminium and calcium products will also impair the absorption of oral iron but the increasing popularity of intravenous iron seems to be lessening the importance of this problem.
Doses of all binders are given with meals, and titrated upwards against serum phosphate. It is important for pharmacists to stress and promote compliance with what are usually large tablets with a variety of gastrointestinal adverse effects. In addition, pharmacists should emphasise the need to take the tablets with food and to maintain dietary restrictions.
Aluminium hydroxide
Aluminium hydroxide was the only binder available for many years, and it is still both the cheapest and the most effective binder of phosphate. However, aluminium also causes a reduced response to erythropoietin and darbepoetin, and long term fears about bone and brain toxicity have reduced its popularity, especially in children.
The most recent K/DOQI guidelines [2] restrict aluminium use to eight weeks’ therapy at a time.
Calcium preparations
As a result of fears about aluminium, calcium preparations (in particular calcium carbonate [Calcichew, Adcal], and calcium acetate [Phosex]) have become the preferred products in the UK, although only licensed for dialysing patients. But while the administration of large amounts of elemental calcium to patients may well reduce serum phosphate, increased calcium load can contribute to a calcium-phosphate product that remains high, leading to poor long-term cardiovascular outcomes. It would seem wise to monitor calcium-phosphate product values a sclosely as those of phosphate, and the K/DOQI guidelines say that elemental calcium load should ideally be held below 2g/day, which may not be possible if phosphate is to be controlled.
The search has been, therefore, for a phosphate binder that does not contribute calcium or aluminium loads to a patient and, hopefully, reduces calcification and cardiac death risks, although this latter claim has not yet been proved prospectively.
Non-calcium and non-aluminium treatments
The only licensed non-calcium and non-aluminium product currently available in the UK is sevelamer (marketed as Renagel tablets), anion-exchange gel that binds phosphate and cholesterol in the gut. Sevelamer is not a particularly powerful phosphate binder and typically costs several thousand pounds per patient annually. For these reasons, many units would use sevelamer in conjunction with calcium or aluminium products. Another non-calcium and non-aluminium binder, lanthanum carbonate (Fosrenol) is expected to be launched in the UK by Shire Pharmaceuticals this year.
Lanthanum is a rare-earth metal that readily forms an insoluble phosphate with minimal systemic absorption, and offers another option in the range of binders, although it is unlikely to be cheap.
The choice of phosphate binder is thus restricted to a fairly small range of medical products. In choosing a regimen or writing a protocol the ri sk-benefit balance and cost of each product need to be considered. TheK/DOQI guidelines suggest, for example, using the more expensive non-calcium binders when:
- Serum calcium is above normal reference range (risk of elevating calcium and calcium-phosphate product further)
- There is severe vascular or soft tissue calcification
- PTH is “low” (below two times the upper limit of the normal reference range) and there is a risk of suppressing PTH further, causing low-turnover bone disease
Calcimimetics
In the absence of a vitamin D sterol that can suppress PTH without elevating calcium, a new class of drugs, calcimimetics, offers a way forward. Cinacalcet (marketed this year by Amgen as Mimpara) is the first calcimimetic that binds to the calcium sensing receptor on the parathyroid glands causing a conformational change which increases sensitivity to circulating calcium.
Thus, PTH is suppressed by lower levels of serum calcium. Clinical experience so far suggests that cinacalcet is effective at reducing PTH, serum calcium and serum phosphate but, due to a still-emerging evidence base and high cost, its exact place in therapy has yet to be defined. Most units would typically use this drug in patients whose PTH levels remain high, despite prescribing the maximum pulsed alfacalcidol dose that a patient can tolerate without producing hypercalcaemia. Cinacalcetis started at a dose of 30mg daily and this is increased slowly, titrating against serum PTH.
Few patients seem to require the maximum licensed dose of 180mg. There is currently little evidence to support the use of cinacalcetin tertiary hyperparathyroidism — it may be less useful once regulatory control over the parathyroid glands has been lost.
Summary
The availability of vitamin D sterols and phosphate binders has meant that in recent years hypocalcaemia and uncontrolled hyperphosphataemia have become uncommon in severe renal impairment. However, it has proved less easy to correct the underlying hyperparathyroidism and calcium-phosphate product problems that are important for maintaining long-term health.
The availability of cinacalcet and new binders opens up exciting new possibilities.
John Sexton, MSc, MRPharmS, is principal pharmacist lecturer-practitioner at the Royal Liverpool and Broadgreen University Hospitals NHS Trust and Liverpool John Moores University Marc Vincent, BPharm ,MRPharmS, is senior renal services pharmacist at the Royal Liverpool and Broadgreen University Hospitals NHS Trust
Action: practice points
Reading is only one way to undertake CPD and the Society will expect to see various approaches in a pharmacist’s CPD portfolio.
1. Observe the phosphate binders and vitamin D analogues you dispense and identify local patterns. Understand the rationale for their use and the problems they might cause. Pharmacists on the renal unit should be happyto discuss local policy with you.
2. Identify whether your renal patients taking phosphate binders understand the importance of compliance and timing. In addition, do they restrict their dietary phosphate as directed bya renal dietician?
3. Make a list of foods that are high in phosphates
Evaluate
For your work to be presented as CPD, you need to evaluate your reading and any other activities. Answer the following questions: What have you learnt? How has it added value to your practice? (Have you applied this learning or had any feedback?) What will you do now and how will this be achieved?
Resources
- Renal Association. Haemodialysis and peritoneal dialysis: nutritional and biochemical standards. Treatment of adults and children with renal failure: standards and audit measures. Available at: www.renal.org/Standards/RenalStandards_2002b.pdf (accessed on 30 March 2005).
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. American Journal of Kidney Disease. Available at: www.kidney.org/professionals/kdoqi/guidelines_bone/Guide6.htm (accessed on 30 March 2005).
- Department of Health. Management of medicines: a resource document for aspects specific to the National Service Framework for Renal Services 2003. Available at: www.dh.gov.uk/assetRoot/04/08/11/34/04081134.PDF (accessed on 30 March 2005)