Zephyr / Science Photo Library
After reading this article, you should be able to:
- Understand the role of a pharmacist in renal cell carcinoma;
- Understand staging and treatments in metastatic renal cell carcinoma;
- Identify common side effects of treatment.
Renal cancer (RC) is the 7th most common cancer and the 13th most common cause of cancer death in the UK[1]. Since the 1990s, the incidence of RC in the UK has increased by 88% (94% in females, 77% in males), and there are now approximately 13,300 new cases and 4,600 RC deaths each year. Incidence rates are projected to rise by a further 26% between 2014 and 2035[1].
This rising incidence has led to a corresponding 73% increase in RC deaths in the UK compared with the 1970s; however, survival has improved owing to significant advances in the treatment of metastatic disease[1]. Pharmacy teams are increasingly likely to be involved in the management of patients with RC and it is important to have an understanding of systemic treatments for RC and their potential toxicities.
The risk of developing RC depends on many factors — including age, genetics and exposure to risk factors — but it is estimated that approximately 34% of RC is preventable[1].
Risk factors for RC include:
- Smoking: increases risk by 30–35%, and accounts for 13% of RC[2]. Increased cigarette usage is associated with more advanced disease at presentation[3];
- Obesity: accounts for 24% of RC[4];
- Deprivation: linked to around 1,100 cases per year — female rates are 40% higher in the most deprived compared with the least, and males are 17% higher[1];
- Race: in England, between 2013 and 2017, RC was found to be more common in white populations[1];
- Hypertension: each 10mmHg increase in systolic and diastolic blood pressure is associated with a 10% and 22% increase in risk[5];
- Exposure to toxic agents, such as cadmium, asbestos, petroleum products and cytotoxic chemotherapy[6,7];
- Genetics: risk increases if a person has a first-degree relative with RC[8];
- Other medical conditions: history of kidney stones, chronic hepatitis C infection or chronic kidney disease[9–11].
Public health messaging and campaigns to reduce smoking, obesity and blood pressure can contribute to reducing the incidence of RC, as well as other diseases.
Owing in part to increased use of abdominal imaging, 50% of renal masses are detected incidentally[12]. Two thirds of patients who present with localised RC are amenable to curative surgery (one third of these will eventually develop distant metastases) and a third have metastatic disease at presentation[13].
Many patients are asymptomatic at diagnosis. Symptoms are vague and can also indicate other conditions/cancers, making differential diagnosis necessary.
Signs and symptoms of RC include[14]:
- Haematuria;
- Flank/groin pain and/or a palpable abdominal mass;
- Anorexia, cachexia, weight loss;
- Malaise;
- Signs/symptoms of anaemia (e.g. pallor, weakness, dyspnoea);
- Fever (may present as fever of unknown origin);
- Symptoms of hypercalcaemia (e.g. confusion, nausea, fatigue, polyuria);
- Signs/symptoms of metastatic disease (e.g. focal bone pain, cough/wheeze, neurological symptoms);
- Hypertension;
- Lymphadenopathy;
- Lower extremity oedema.
The pharmacy team is ideally placed to aid the early diagnosis of cancers and to refer urgently[15]. It is also ideally placed to support patients during the course of their treatment and signpost them to charitable organisations and other sources of help (see ‘Useful resources‘).
Pathophysiology of renal cancer
Renal masses can be simple renal cysts requiring no treatment or benign renal lesions (angiomyolipomas or oncocytomas) or renal cell carcinomas (RCCs)[16]. RCCs are the most common cause of RC and these will be the main theme of this article[13].
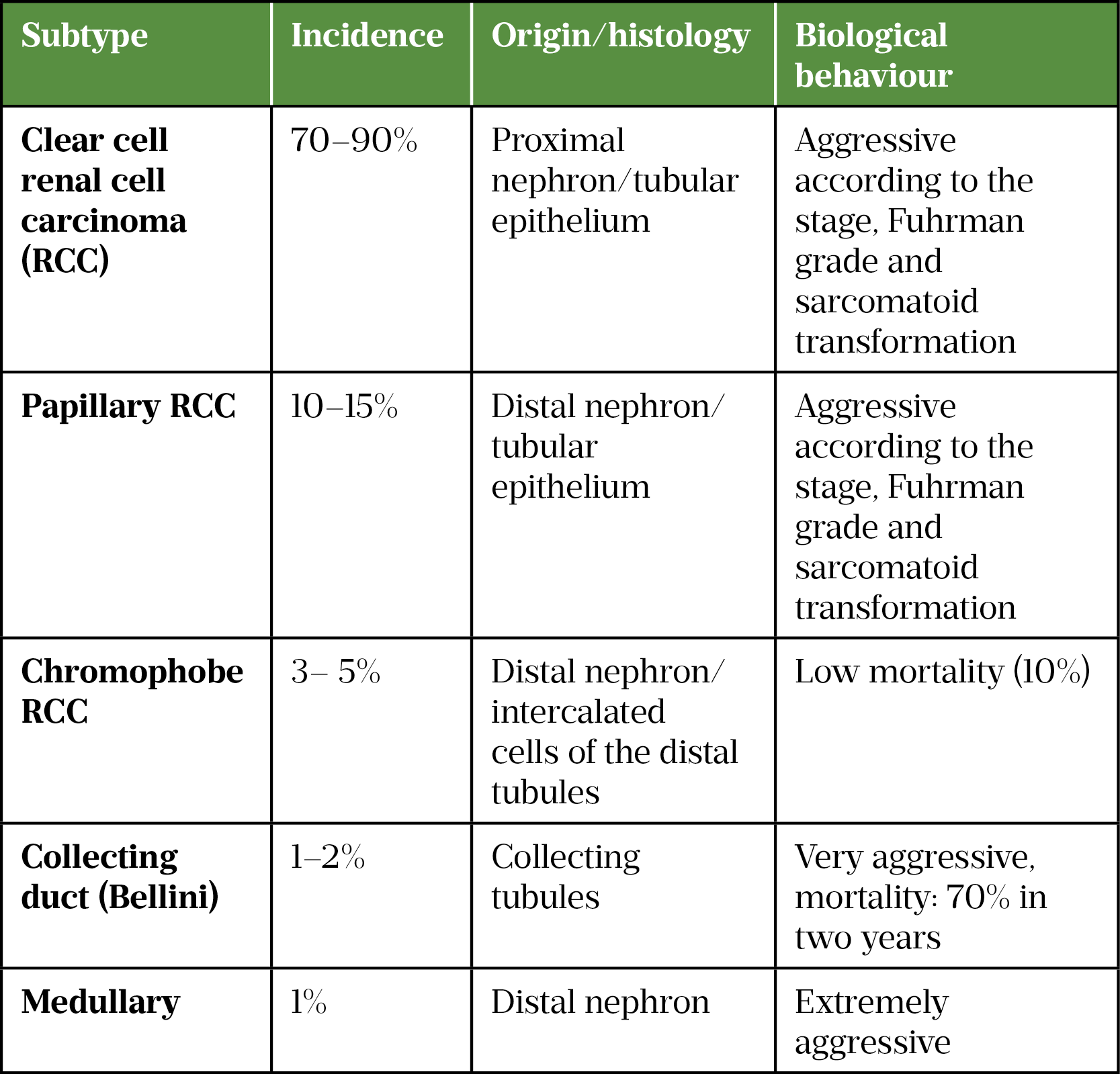
RCC is the most common malignancy that originates from the renal cortex and is a heterogenous group of tumour types, with each having different histology, clinical course and gene involvement (see Table 1[17–19]).
Clear cell RCC (ccRCC) is the most common subtype and is more likely to present at an advanced stage[18]. The name clear cell refers to the cells appearance under a microscope.
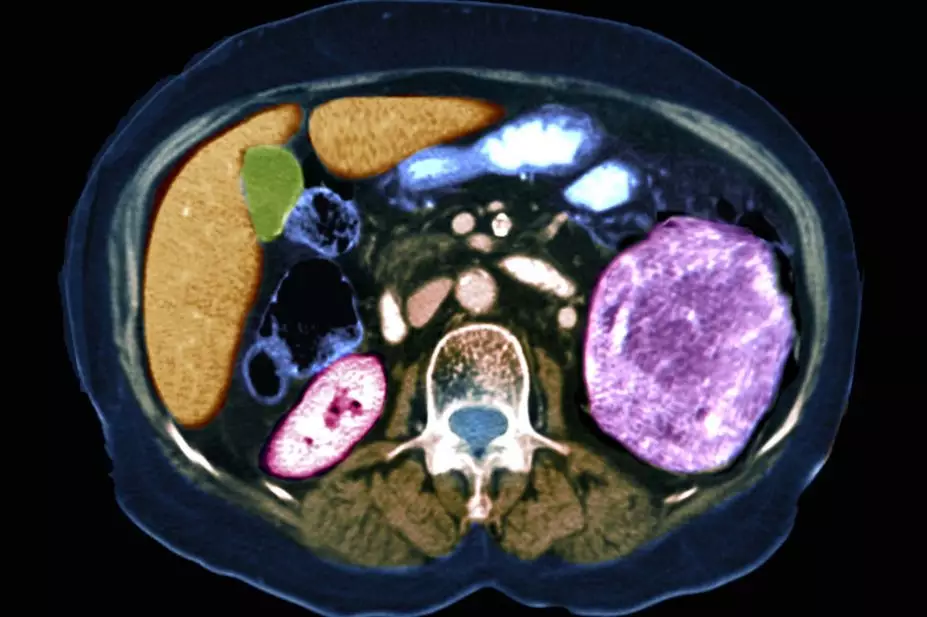
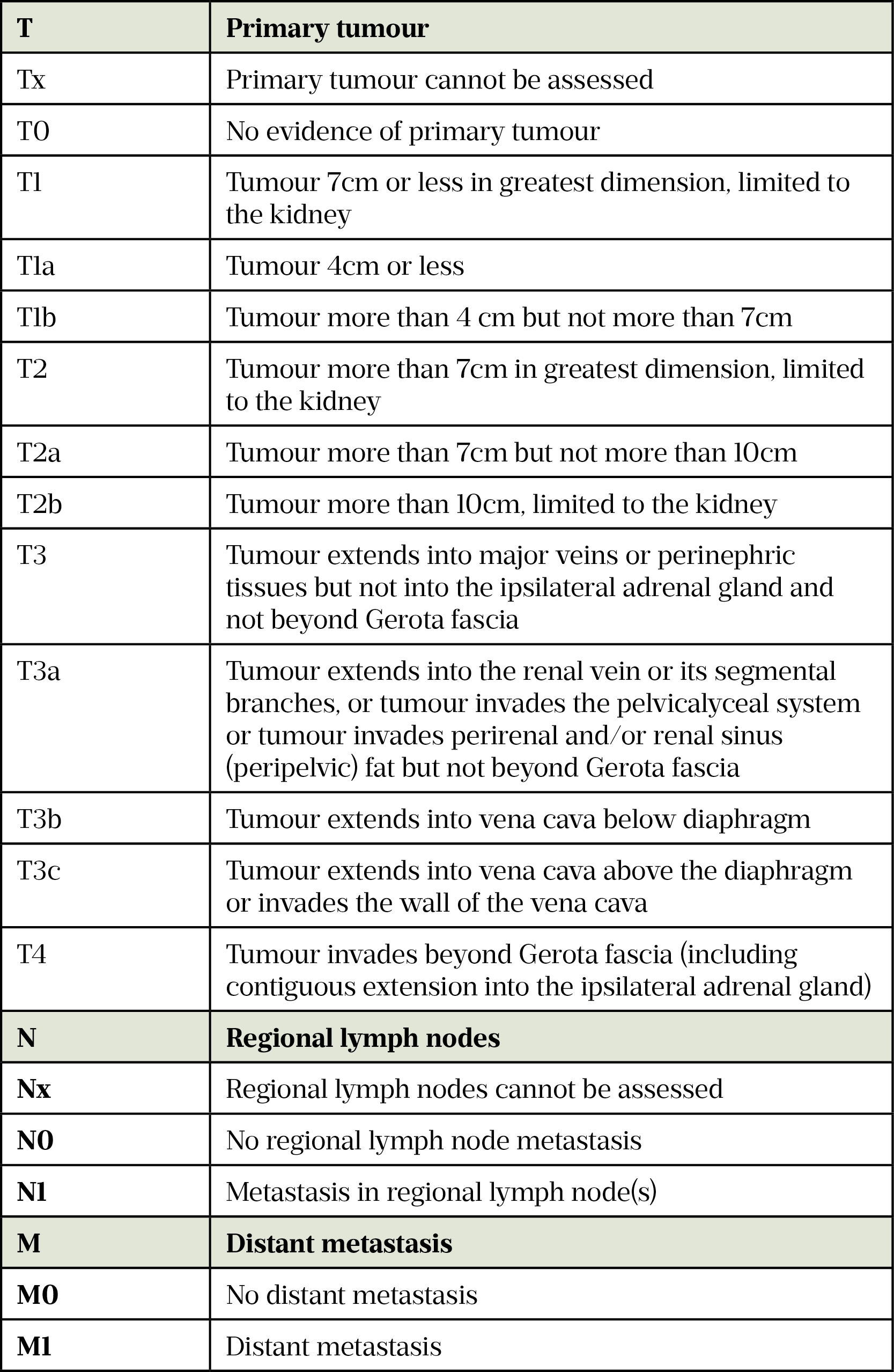
Investigations for suspected RCC include a CT scan of the chest, abdomen and/or pelvis, which allow for tumour staging. Staging involves assessing the size and position of the primary tumour (T), lymph node (N) involvement and the presence of distant metastasis (M) (see Table 2[12,20]). Depending on local guidelines, patients with metastatic disease may also have a CT scan of the head to look for brain metastases. Prognosis depends on the tumour stage (see Table 3[12,20,21]).
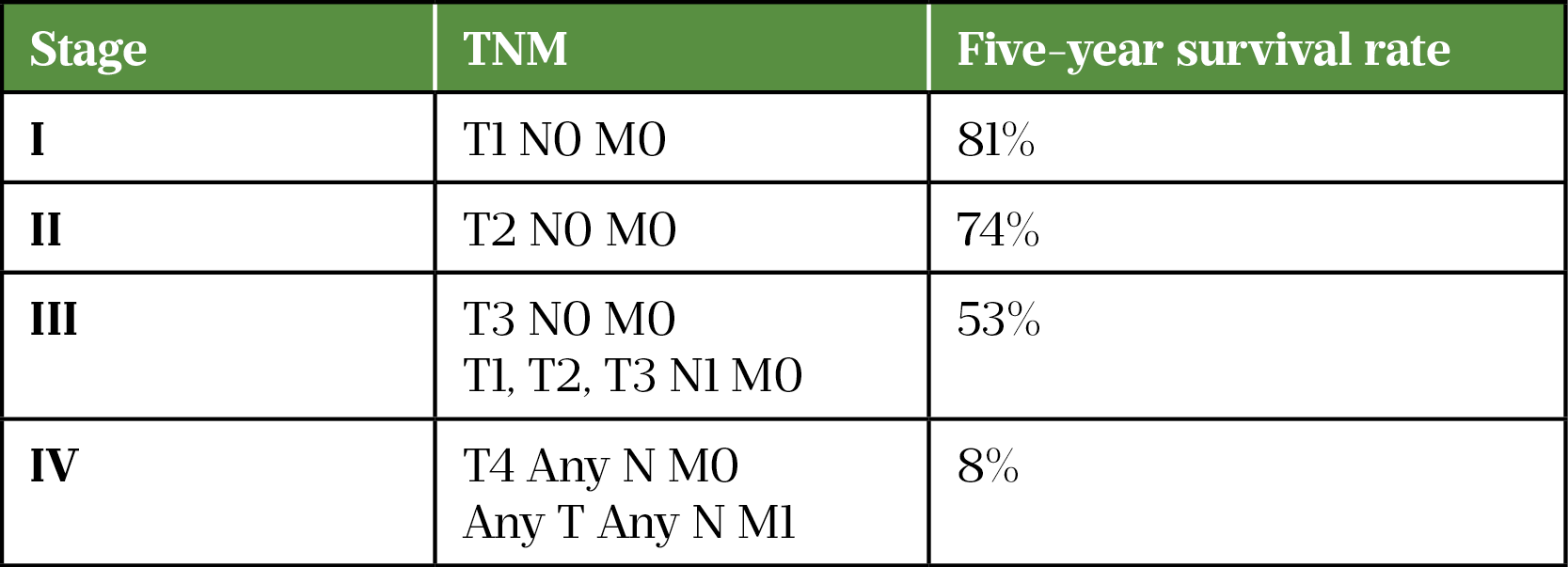
Box 1 provides an example of a kidney tumour progressing through the four stages.
Box 1: The four stages of a kidney tumour
Stage 1: Tumour size ≤7cm in kidney
Stage 2: Tumour size >7cm in kidney
Stage 3: Tumour involving veins or one or more lymph nodes; within Gerota fascia
Stage 4 : Tumour extends past Gerota fascia; or any adrenal gland involvement
Treatment targets in renal cell carcinoma
The majority of RCCs are sporadic, arising from gene mutation. A particular gene called von Hippel-Lindau (vHL) is believed to be partly responsible for the development of RCCs, with a vHL mutation found in 80% of ccRCC[22,23]. This gene is also defective in vHL syndrome, a genetic condition that increases the risk of developing ccRCC by up to 60%[24]. An estimated 3–8% of RCCs are caused by inherited gene alterations[24,25].
The vHL gene is involved in the regulation of proteins called hypoxia inducible factors (HIF) 1 and 2, which play an important role in the normal cellular response to hypoxic conditions. Inherited mutations of the vHL gene can affect the production of the vHL protein, leading to the build up of HIF in cells. Excessive HIF can then promote invasive growth of cancer cells and excessive formation of new blood vessels (angiogenesis). These pathways are the target for oral agents used to treat RCCs[26].
RCC tumour growth and angiogenesis are mediated through vascular endothelial growth factor (VEGF), HIF and mTOR pathways, and targeting these pathways inhibits tumour cellular metabolism, cell growth and vascular network expansion[27,28]. In metastatic RCCs, the VEGF pathway can be targeted with oral small-molecule VEGF receptor tyrosine kinase inhibitors (VEGF TKI), including axitinib, cabozantinib, lenvatinib, pazopanib, sunitinib and tivozanib[29]. Each TKI targets VEGF, but also targets other pathways and receptors.
Additionally, genetic changes in mammalian target of rapamycin (mTOR) pathway proteins have been identified in up to 28% of RCCs and are targets for mTOR receptor inhibitors, such as everolimus[29,30].
RCC cancer cells are also able to ‘switch off’ the immune system, allowing cancer cells to avoid immune system attack. Tumour cells ‘hijack’ certain immune checkpoint pathways (such as programmed death-1 [PD-1]/programmed death ligand-1 [PD-L1]) that would normally lead to activation of T-cells specific for tumour antigens and tumour cell destruction[31].
In normal health, the immune system T-cell activation is initiated by T-cell receptors that recognise antigens (such as cancer cells), a process controlled by a balance between inhibitory (immune checkpoints) and stimulatory signals. Consequently, the immune system can maintain self-control or self-tolerance (i.e. preventing autoimmune responses) while protecting tissue from damage when responding to a pathogen or cancer cell[31].
This pathway has a role in RCCs, with 23% of ccRCCs, 10% of papillary RCCs and 5.6% chromophobe RCCs showing PD-L1 expression[32,33]. The PD-L1 receptors on the surface of the RRC cells block the immune cells from destroying the cancer cell and forms an ‘immune evading shield’, which protects cancer cells from being attacked by the immune system[34]. Currently licensed anti-PD-1/PD-L1 treatments include avelumab, pembrolizmab and nivolumab.
Additionally, cytotoxic T lymphocyctes antigen 4 (CTLA-4) is a T-cell receptor that, when activated, suppresses the action of T-cells and prevents over activity or unwanted immune responses. However, a protein found on the surface of cancer cells can block CTLA-4 and unintentionally allow cancer growth. Ipilumumab is an anti-CTLA-4 antibody, which increases the activity of T-cells and suppresses the activity of cancer cells[34].
Management of renal cell carcinoma
The management of RCC will depend on whether the disease is localised or metastatic.
Localised disease
If RCC is confined to the kidney (no metastatic disease) then management is through curative surgery. The preferred options are:
- T1 tumour (<7cm): partial nephrectomy[12,35];
- T2 tumours (>7cm): laparoscopic nephrectomy[12];
- Locally advanced RCC (T3 or T4): open or laparoscopic nephrectomy[12].
Active surveillance is an option in patients who are not suitable for surgery (e.g. older patients with significant comorbidities or patients with a short life expectancy).
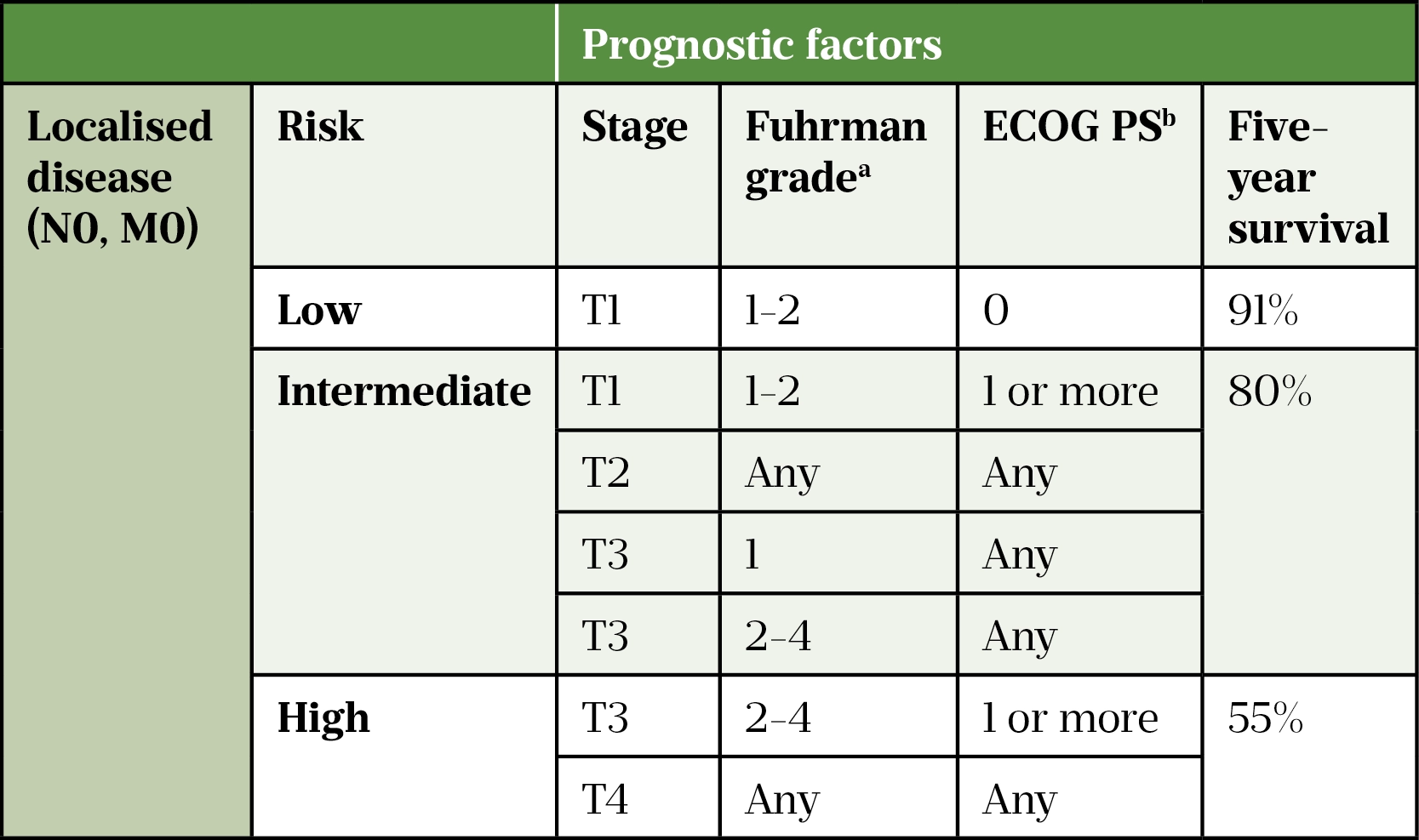
a Fuhrman grading system:
Grade 1 or low-grade cells are usually slow-growing, look quite similar to normal cells, tend to be less aggressive and are less likely to spread; grade 2 or intermediate grade cells grow more quickly, look abnormal, are moderately aggressive and could spread; grade 3 or high-grade cells are likely to grow more quickly, look very abnormal, tend to be more aggressive and are more likely to spread; grade 4 or high-grade cells look very abnormal, grow very quickly, are extremely aggressive and are very likely to spread
b Eastern Cooperative Oncology Group (ECOG) performance status
High-risk localised disease has a five-year survival rate of 55% (see Table 4[12,36,37]). Trials using TKIs in high-risk localised disease post nephrectomy have not shown any significant survival benefit[37,38].
Using immunotherapy post nephrectomy appears promising. The National Institute for Health and Care Excellence (NICE) is reviewing the use of adjuvant (post-surgery) pembrolizumab on the basis of the KEYNOTE-564 study, which compared adjuvant pembrolizumab (200mg intravenously once every 3 weeks for up to 17 cycles) with placebo in ccRCC at high risk for recurrence after nephrectomy[39,40]. The results show that pembrolizumab was associated with improved disease-free survival (DFS): 77% compared to 68% in the placebo group (HR 0.68, 95% CI 0.53-0.87) and improved overall survival (OS) at 24 months: 97% versus 94% (HR 0.54, 95% CI 0.30-0.96).
However, the OS results are immature and uncertainties remain regarding the optimal selection of patients for adjuvant therapy while risking potentially life threatening/changing immune-related side effects. The use of immunotherapy post nephrectomy might also impact on subsequent treatment options for patients who relapse with metastatic disease.
Metastatic disease
Patients with a resectable primary RCC and a solitary or limited number of resectable metastases may be considered for surgery. Cytoreductive nephrectomy, or debulking surgery, can also be considered for patients with metastatic RCC who have an International Metastatic RCC Database Consortium (IMDC) score of 0–1 (see Table 5), low disease burden and good performance status[41].
Risk stratification of inoperable metastatic renal cell carcinoma
Patients with inoperable disease who are candidates for systemic therapy will require a biopsy (of either the primary renal tumour or a site of metastatic disease) to confirm the histological subtype as well as blood tests to inform prognosis and choice of treatments.
Patients with metastatic RCC are stratified to guide treatment choice and estimate OS in patients treated with systemic therapy (see Table 5). The Memorial Sloan Kettering Cancer Center risk model was the initial gold standard, but the IMDC risk model is now more commonly used[42–44]. Approximately 25% of patients are in the IMDC favourable-risk group, 50% are intermediate risk and 25% are poor risk[45].
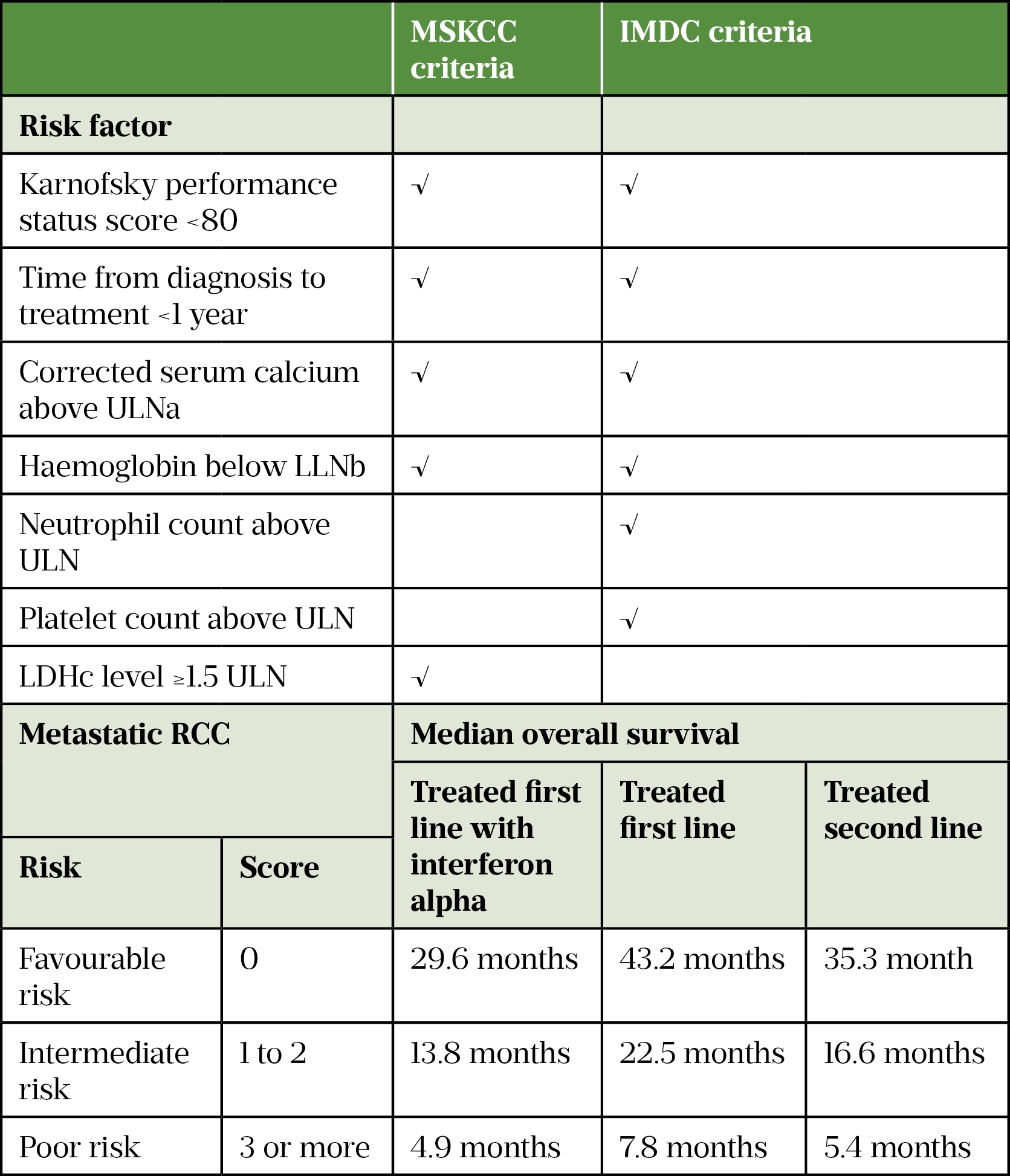
Performance status is a factor in prognosis and in determining the best treatment for a patient with cancer. Performance status is a score that estimates the patient’s ability to perform certain activities of daily living (ADLs) without the help of others. These ADLs include basic activities, such as getting dressed, eating and bathing, as well as more complex activities such as cleaning the house and working a regular job[47].
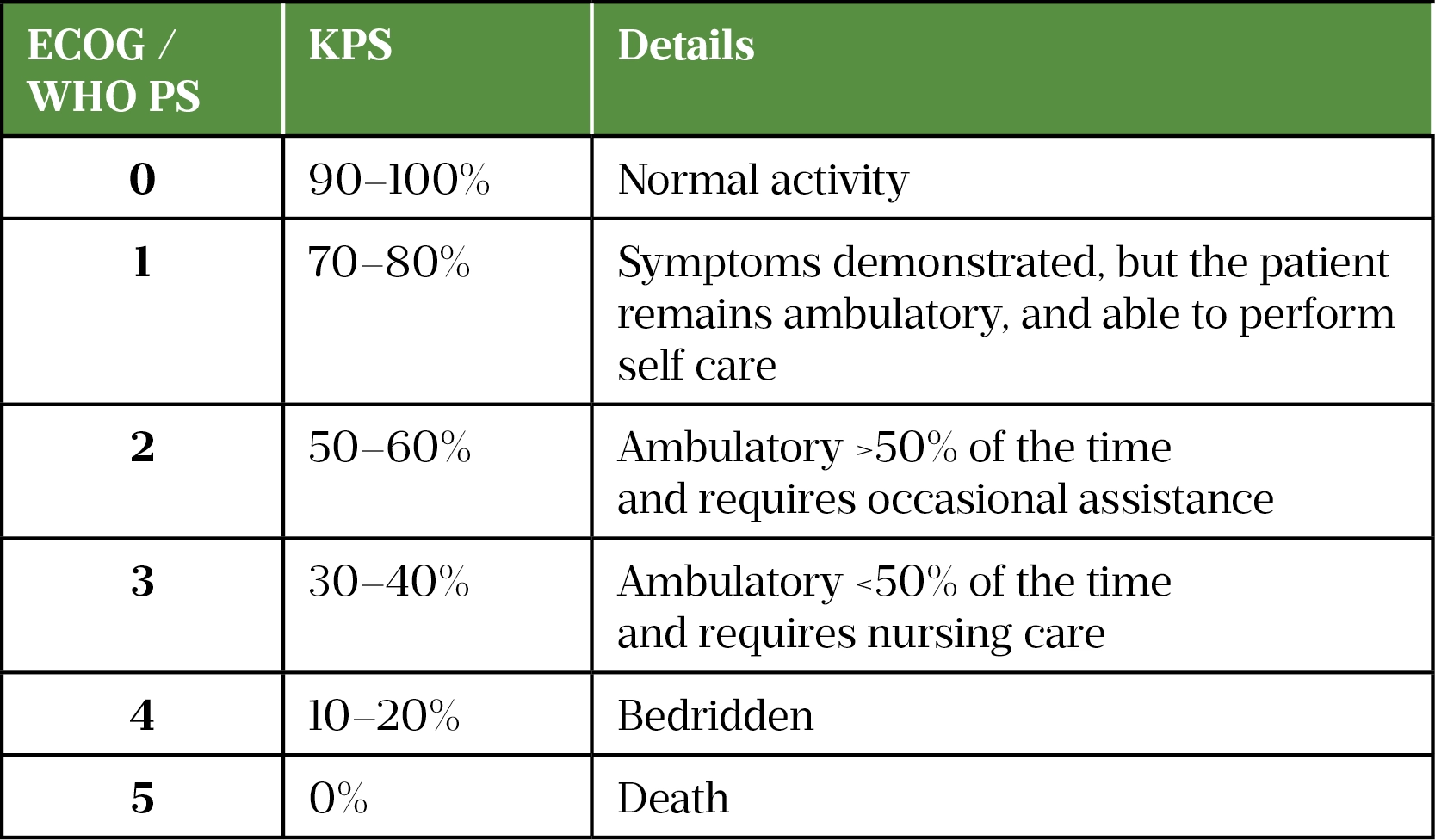
The commonly used performance status scoring systems are:
- Eastern Cooperative Oncology Group Performance Status (ECOG PS);
- Karnofsky Performance Status (KPS);
- World Health Organization Performance Status (WHO PS).
Table 6 provides an approximate conversion guide between the ECOG/WHO and KPS systems[48].
First-line treatment for metastatic disease
Metastatic RCC is inherently resistant to chemotherapy and radiotherapy. Historically, immunotherapeutic agents (interleukin-2 and IFN-α) showed modest objective response rates (ORR) and were superseded by oral VEGF TKIs treatments[49,50]. Between 2005 and 2018, pazopanib or sunitinib were the standard first-line treatment for metastatic RCC until doublet immune checkpoint inhibitors (ICI) with ipilimumab plus nivolumab followed by nivolumab monotherapy and combination ICI with VEGF TKI completely changed the treatment pathway[51–60].
There are multiple first-line treatment options (see Figure 1[41]) for patients with metastatic clear cell RCC:
- Combination immunotherapy (anti-PD-L1) with VEGF TKI (avelumab-axitinib or lenvatinib-pembrolizumab or pembrolizumab-axitinib or nivolumab-cabozantinib[56–60];
- Doublet ICI with ipilimumab (an anti-CTLA-4 ICI) plus nivolumab followed by nivolumab monotherapy[53–55];
- Single agent oral VEGF TKI (either tivozanib, pazopanib, sunitinib or cabozantinib)[61–66];
- Combination ICI (anti-PD-L1) with VEGF TKI (avelumab-axitinib or lenvatinib-pembrolizumab or pembrolizumab-axitinib or nivolumab-cabozantinib[56–60].
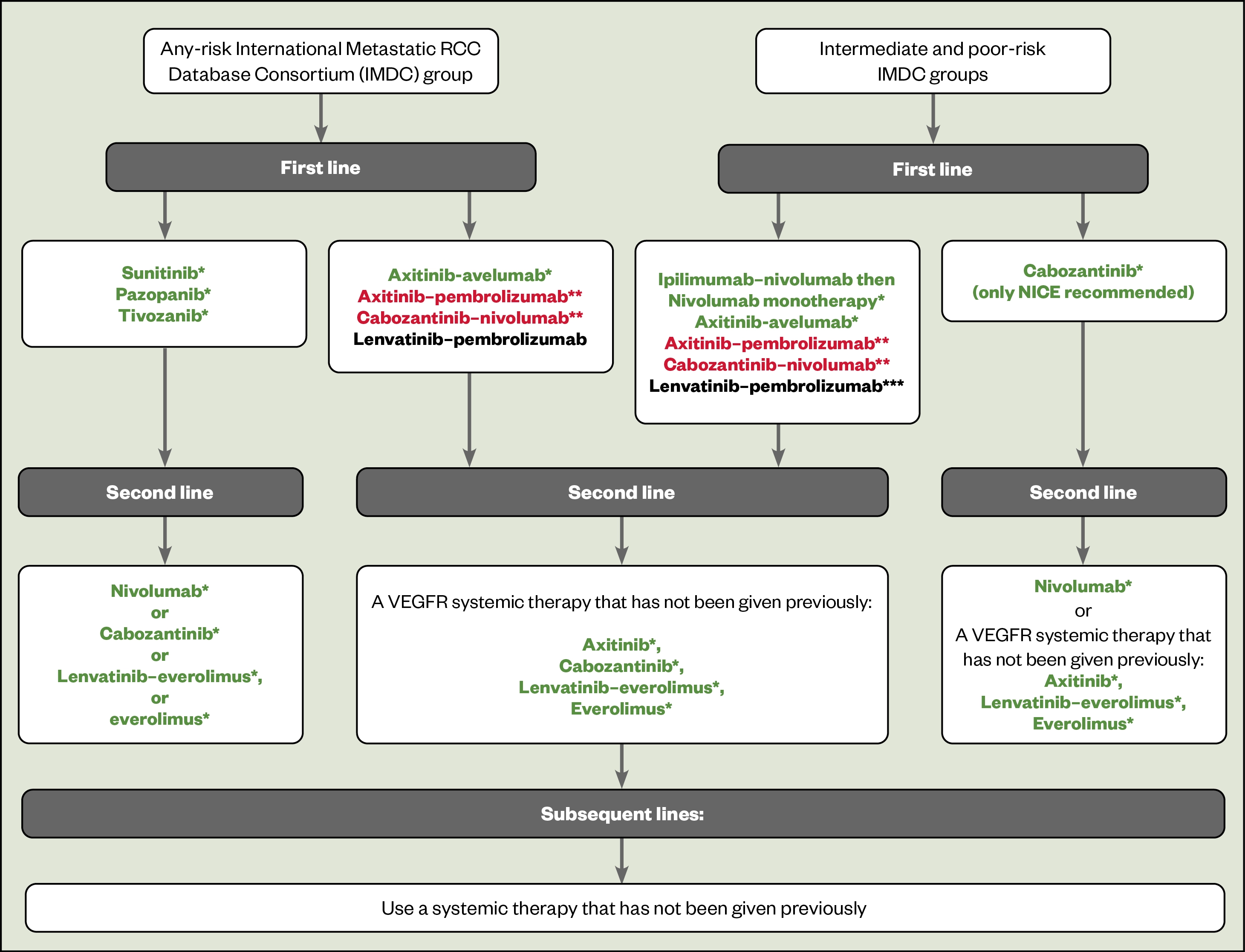
In clinical trials, the use of combination ICI with VEGF TKI tended to improve disease progression or death from any cause (progression-free survival [PFS]), OS and response rates when compared with a single agent oral VEGF TKI. This makes the combination ICI with VEGF TKI a better first choice in treatment-naive patients with any IMDC risk metastatic ccRCC.
Single agent VEGF TKI are alternatives when an ICI is not suitable or contraindicated, such as owing to risk of immunotherapy-related toxicities.
Between 2005 and 2018, pazopanib and suntinib were the standard first-line treatment for metastatic RCCs. Pazopanib, sunitinib or tivozanib are all approved by NICE for treatment-naive patients in any IMDC risk group with metastatic ccRCC[51,52,67]. VEGF TKIs improve PFS and ORR for treatment-naive patients, but not OS[61,62,68].
Currently, the only NICE-approved ICI/VEGF combination is axitinib-avelumab, which was approved for use within the Cancer Drugs Fund as an option for untreated advanced RCC, even though OS data are not currently available[69]. The Scottish Medicines Consortium (SMC) has approved axitinib-avelumab, axitinib-pembrolizumab and cabozantinib-nivolumab[70–72].
Treatment-naive intermediate or poor risk (IMDC score ≥1) metastatic ccRCC account for 75% of patients[45]. Treatment options are:
- Doublet ICI with ipilimumab (an anti-CTLA-4 ICI) plus nivolumab followed by nivolumab monotherapy[53–55];
- ICI/VEGF TKI combination[56–60];
- Cabozantinib (an oral TKI that inhibits VEGF and also MET and AXL receptors)[65,66].
Ipilimumab-nivolumab (given for four cycles followed by nivolumab monotherapy) has been compared with sunitinib in treatment-naive patients and transformed the standard of care for patients with intermediate/poor risk metastatic RCC[53]. This study demonstrated that in patients with intermediate/poor risk metastatic RCC, ipilimumab-nivolumab improved PFS and OS.
OS gain was 20 months, with some patients achieving long-term durable responses (PFS at four years were 32.7% for ipilimumab-nivolumab versus 12.3% for sunitinib)[54,55]. This remarkable gain in OS and durable responses seen with ipilimumab-nivolumab has seen a change in practice for treatment naive intermediate/poor risk IMDC groups and led NICE and the SMC to recommend its use as a first-line option for patients with an intermediate/poor risk IMDC score[73,74].
Ipilimumab-nivolumab is not indicated for patients with favourable risk disease as it did not improve OS when compared to sunitinib[53].
ICI/VEGF TKI combinations are also options for patients with intermediate/poor risk disease, and are associated with a higher ORR than ipilimumab-nivolumab (e.g. pembrolizumab-lenvatinib has an ORR of 71%, whereas the ORR for ipilimumab-nivolumab is 42%). ICI/VEGF combinations are therefore an attractive option for patients with a high burden of disease or disease at critical sites, as these patients may not subsequently be fit for second-line treatment. The toxicity profile of ICI/VEGF combinations also differs from ipilimumab-nivolumab and this also influences decisions regarding treatment.
Cabozantinib is an alternative in IMDC intermediate or poor-risk metastatic ccRCC in those who cannot receive an ICI. Cabozantinib was compared to sunitinib and showed that cabozantinib significantly improves ORR and PFS (8.6 versus 5.3 months), but not OS which makes cabozantinib a less attractive option. Cabozantinib is recommended by NICE in metastatic renal cell carcinoma in IMDC intermediate/poor risk patients[65,66,72,75].
Subsequent treatment for metastatic disease
Second and third-line treatment (see Figure 1) depends on what treatments the patient has previously received. If they received:
- First-line ICI: consider VEGF TKI second line;
- First-line VEGF TKI: consider single agent nivolumab or alternative VEGF TKI;
- First-line ICI in combination with VEGF TKI: consider lenvatinib-everolimus or cabozantinib.
Cabozantinib 60mg daily has been shown in metastatic RCC patients who progressed after VEGFR-targeted therapy to improve PFS and OS (21.4 versus 16.5 months, HR 0.66, p=0·00026) when compared with everolimus[76].
Single agent IV nivolumab has demonstrated benefit after one or more VEGF TKIs. The CheckMate 025 study compared nivolumab (IV 3mg/kg every two weeks) with everolimus (10mg daily) and showed improved ORR (25% compared to 5%)[77]. Five-year follow-up data demonstrated nivolumab maintained an OS benefit of 25.8 months with nivolumab compared to 19.7 months with everolimus[78].
A small randomised phase II open label study compared lenvatinib (18mg/day) in combination with everolimus (5mg/day) against single agent everolimus (10mg/day) and showed that patients who received lenvatinib-everolimus had a significantly longer PFS (14.6 versus 5.5 months). Toxicity is a concern with lenvatinib-everolimus with severe toxicities occurring in 71% of patients who received combination treatment compared to 50% of patients who received everolimus[79].
Both axitinib and everolimus have shown a modest improvement in PFS only, but there was no significant difference in OS[80,81].
The use of third and fourth-line tivozanib in ccRCC improved PFS and was better tolerated than sorafenib[82]. However, it should be noted that the formulation of tivozanib used is not licensed in the UK.
Patient parameters for renal cell carcinoma treatment options
The choice of treatment depends on the IMDC score and availability of the drugs on the NHS (see Figure 1). Patient parameters are also important in choice of treatment.
From experience, the percentage of patients fit enough for treatment reduces as you go through the lines of treatment, which make choice of first-line treatment important. There may also be person-centred factors to consider, for example some patients may prefer an oral option to an infusion.
Patient comorbidities are also important factors in the choice of therapy. There are no absolute contraindications to the use of ICIs, but you would take a cautious approach in patients with an auto-immune disease or an organ transplant[83–85].
ICI/VEGF combinations are an attractive option owing to high response rates for patients with a high burden of disease or disease at critical sites, as these patients may not subsequently be fit for second-line treatment.
There are no absolute contraindications to the VEGF TKIs[86–91]. However, the risks and benefits of VEGF TKIs would be considered before beginning therapy in patients with comorbidities, such as pre-existing heart failure or prolongation of the QT cardiac wave.
Future treatment options for renal cell carcinoma
Belzutifan (currently unlicensed) is an oral inhibitor of HIF-2α and so represents a novel approach to the treatment of RC. Initial studies have shown promising activity in patients with Von Hippel-Lindau disease (ORR of 49%) and a phase II study of belzutifan in combination with cabozantinib in patients with pretreated metastatic RCC showed a disease control rate of 90%[92,93]. At the time of writing, phase III trials are ongoing in the adjuvant and metastatic settings.
Side effects
All pharmacy professionals need a basic understanding of adverse events with cancer treatments and the need to refer for urgent toxicity assessments. All members of the pharmacy team should have a low suspicion that any patient may have toxicity related to their cancer treatment.
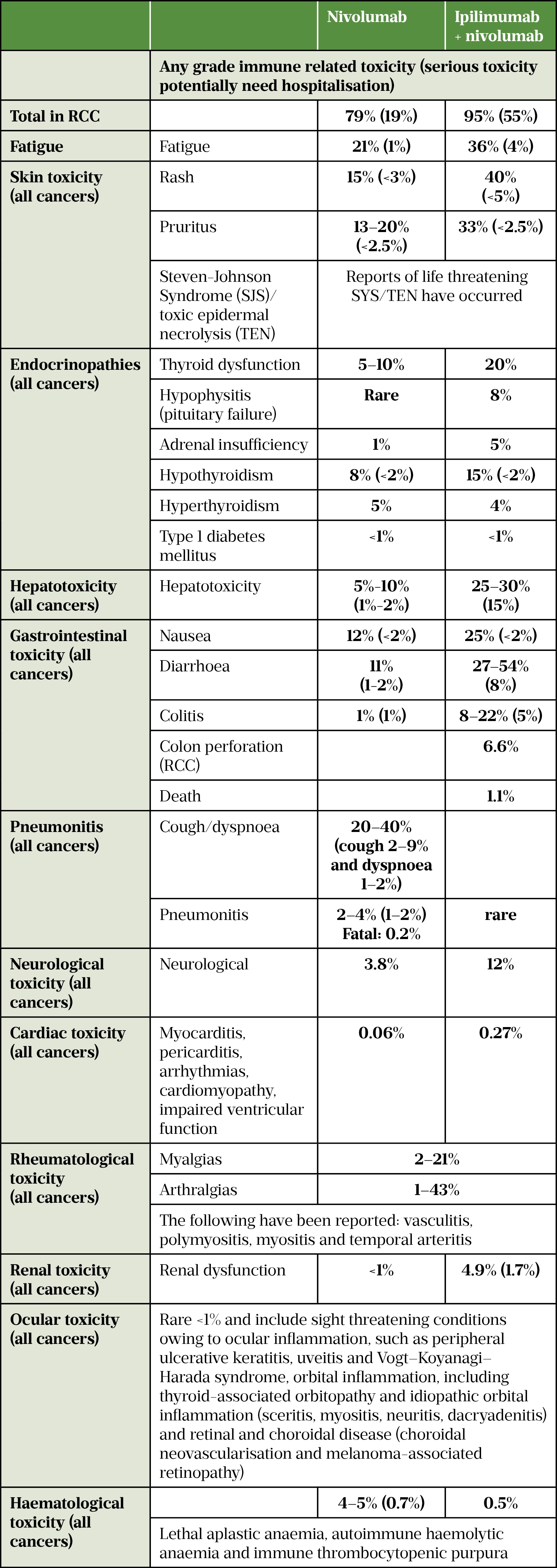
In clinical trials, doublet ICI with ipilimumab plus nivolumab followed by nivolumab monotherapy had less treatment related adverse events than single agent VEGF TKI (93% compared with 97%), but also less severe reactions (46% compared to 63%)[53,54].
The combination of ICI with VEGF TKIs resulted in similar total treatment related adverse events compared with single agent VEGF TKI (>95% in both groups), but combination ICI with VEGF TKIs had more severe events than single agent VEGF TKI[58–60].
Side effects of VEGF TKIs
VEGF TKIs can cause significant and potentially serious adverse events (AEs) with appropriate management being critical to the continuation of treatment while maintaining a good quality of life. Research has shown that 1.5–2.3% of patients have fatal AEs, with haemorrhage being the most common fatal toxicity[94]. VEGF TKIs have a variety of cardiovascular and non-cardiovascular side effects. The cardiovascular side effects are related to the inhibition of blood vessel formation (angiogenesis) within normal tissue.
Different VEGF TKIs have slightly different receptor affinities, which is why they have differing efficacy and side effect profiles. It is common to dose reduce, interrupt or permanently discontinue treatment due to AEs.
The most common/clinically significant AEs are:
- Hypertension, which may require anti-hypertensive therapy. Incidence varies from 22% to 44%[62,66,68,79,80]. Development of TKI-related hypertension is associated with improved outcomes; an analysis of the AXIS study showed that patients who developed hypertension during the first eight weeks of treatment with axitinib had a longer OS than patients whose blood pressure was not elevated[80];
- Decreased left ventricular ejection fraction and heart failure (severe <1%[68]);
- Arterial thromboembolic events, include cerebrovascular accident, transient ischaemic attack and myocardial infarction (incidence 2–3%, fatality rate ≤0.5%)[95–99];
- Venous thromboembolic events (VTE): including deep vein thrombosis and pulmonary embolism VTE incidence, which may be as high as 7% (pulmonary embolism ≤4%, reported fatalities)[95–99];
- Haemorrhage: bleeding occurs in 9.1% of patients (severe bleeds 1.3%) and there is a 1.7 times higher risk of bleeding with a VEGF TKI than controls[100];
- Prolongation of the QT cardiac wave: QT interval prolongation may lead to an increased risk of ventricular arrhythmias including Torsade de pointes (observed in <0.1% with sunitinib[98]).;
- Proteinuria: caused by the involvement of the VEGF pathway in the maintenance of glomerular membranes[101]. Mild, asymptomatic proteinuria occurs in 21–72% of patients and severe proteinuria occurs in 6.5-11% of patients. Discontinuation of the VEGF TKI leads to significant reduction in proteinuria; however, it can persist and patients may develop end-stage renal failure. Although angiotensin-converting-enzyme inhibitors and/or angiotensin receptor blockers seem to be preferred, there have not been trials to support this approach;
- Gastrointestinal side effects, including nausea (incidence 12–44%), vomiting (18–24%, severe up to 4%), diarrhoea (incidence 20–70%, severe up to 11%) and mucositis/stomatitis (incidence: 12–37%, severe up to 5%) are extremely common and although generally mild or moderate in severity, can have a negative impact of quality of life and cause weight loss, dehydration, abdominal pain/cramping and electrolyte disturbance. Usually occurring early in the course of treatment. Cabozantinib has highest incidence 70% (severe 10%) with lenvatinib-everolimus combination (incidence 85%, severe 20%)[62,66,68,79,80,95,99];
- Hepatotoxicity: abnormalities of liver function tests (increases in alanine aminotransferase [ALT], aspartate aminotransferase [AST] and bilirubin) have been frequently observed. Raised ALT/AST: incidence 22–53%, severe up to 12%)[62,66,68,96]. Incidence of hepatic failure is estimated at 0.8%[102];
- Fatigue: incidence 19–64%, severe 2–10%)[62,66,80]. Fatigue can reduce a patient’s quality of life and ability to self-care;
- Palmar plantar erythrodysaesthesia (known as hand and foot syndrome) includes dryness, thickness or cracking of the skin, blisters or rash on the palms of the hands and soles of the feet. Fingers can be painful and so can walking (incidence up to 40%, severe up to 8%)[66,80,98,99];
- Hypothyroidism (incidence 3–20%, severe <0.5%)[61,79,98,99,103]], which is irreversible in nature;
- Impaired wound healing, which occurs in approximately 2% of patients[104]. This has implications prior to planned surgery with manufacturers recommending to stop VEGF TKIs prior to surgery and restart at least two weeks post-surgery, provided adequate wound healing has occurred. However, no formal clinical studies of the effect on wound healing have been conducted. Experience suggests that stopping VEGF TKIs in resistant wounds can lead to healing;
- VEGF TKIs can also cause rare, but potentially fatal adverse events, such as reversible posterior Leukoencephalopathy Syndrome (RPLS) or posterior reversible encephalopathy syndrome (PRES), a rare (<0.1%), potentially fatal neurological disorder that can present with headache, seizure, lethargy, confusion, blindness and other visual and neurologic disturbances. Mild to severe hypertension may be present[86,91].
Side effects of immunotherapy
ICIs target cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), programmed cell death-1 (PD-1), and PD ligand 1 (PD-L1) and work by preventing the receptors and ligands from binding to each other, thereby disrupting signalling so that T cells can recognize and attack cancer cells[105,106]. However, this mechanism can also cause immunotherapy-related AEs (irAE) involving any organ or system of the body by turning the T cell against itself[106]. The non-specific activation of the immune system predominately causes dermatological, endocrine, hepatic, gastrointestinal or respiratory irAE[107] (see Figure 2 and Table 7). International guidance provides comprehensive information for dealing with immune related adverse events[106].
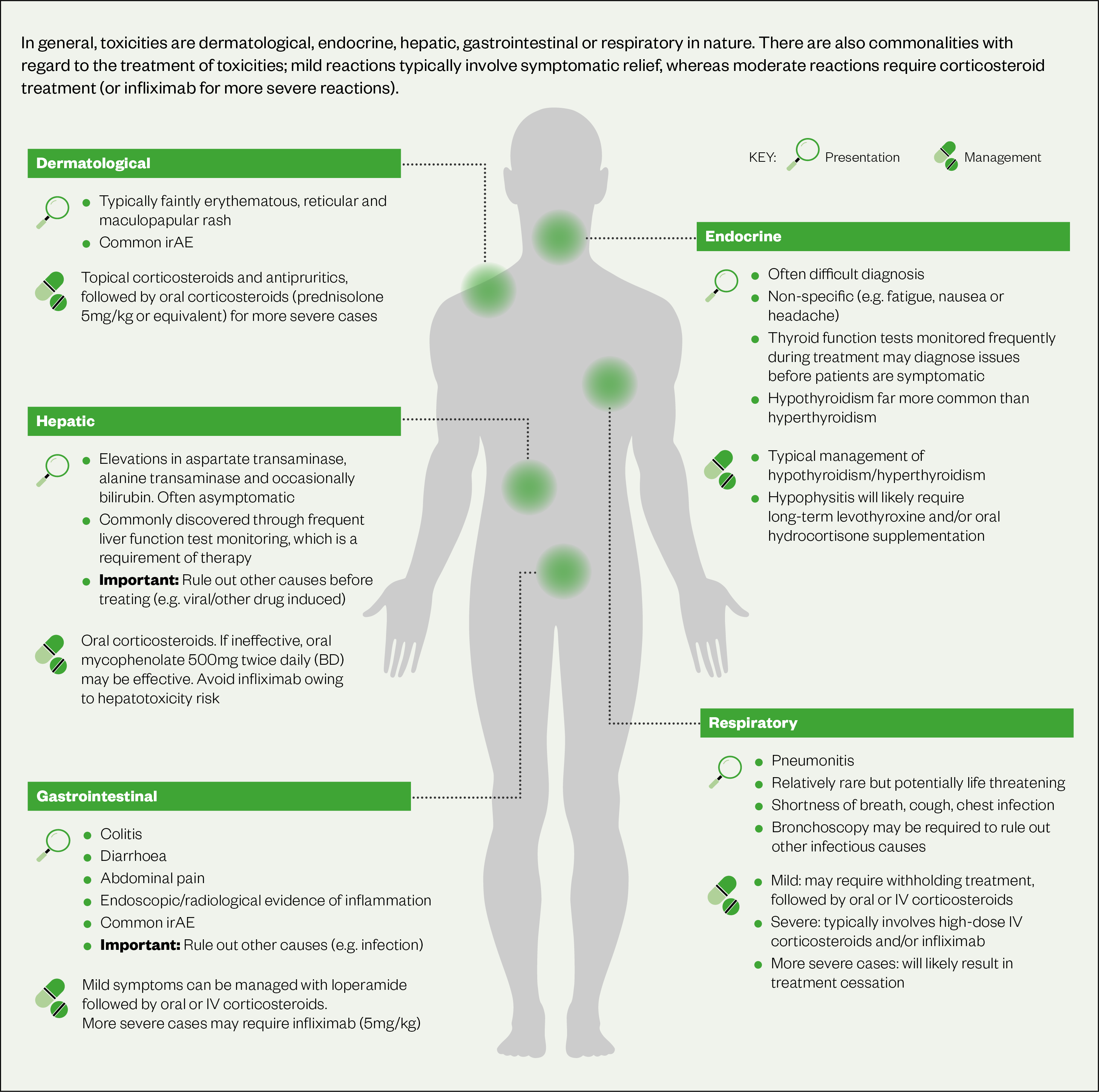
Patient education is important to facilitate prompt reporting of all irAEs. In general, treatment of mild irAEs typically involves symptomatic relief, whereas moderate reactions require corticosteroid treatment and severe reactions require very high doses of corticosteroids (1–2mg/kg prednisolone over at least four to six weeks) and possibly other agents (infliximab, mycophenolate, azathioprine)[105,106]. IrAEs are most likely to develop within the first six months of treatment; however, they can occur at any point during treatment and even after cessation of treatment (beyond 6–12 months)[105,108,109]. IrAEs may cause life-long effects, such as type 1 diabetes mellitus, hypophysitis and hypothyroidism. It is important to have a high suspicion that ICI are causing the adverse effect until proven otherwise.
Side effects of ICI with VEGF TKI combination therapy
The use of ICIs in combination with VEGF TKI agents adds complexity to the management of toxicity, as some of the AEs seen with ICIs are similar to those typically seen with VEGF TKIs (see Figure 3)[111]. Recognising the cause of the AE is important but not always easily possible. For example, diarrhoea occurs in 45% of patients on axitinib monotherapy, but 13% of patients on monotherapy avelumab can have diarrhoea[88,111].
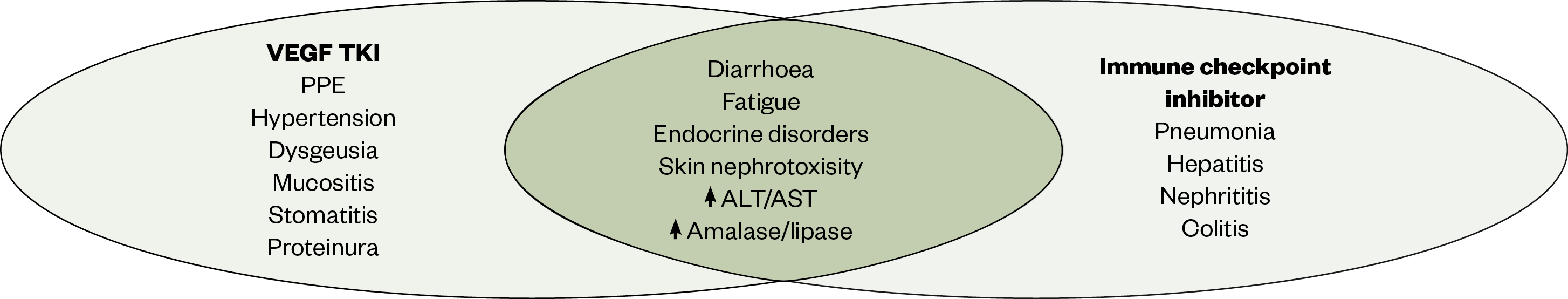
Appropriate assessment of the patient for signs and symptoms that require immediate intervention is essential when using ICI/VEGF TKI combination. If severe irAEs are suspected, it is important to start treatment promptly on the basis of clinical suspicion. In the absence of serious clinical signs, it may be possible to withhold the VEGF TKI and observe for improvement. If no improvement occurs, then consider the possibility of an irAE and treat with steroids. This is easier with axitinib as it has a relatively short half-life (between 2.5 and 6 hours), but is more difficult with drugs such as cabozantinib where the half-life is around 4.5 days[86,88].
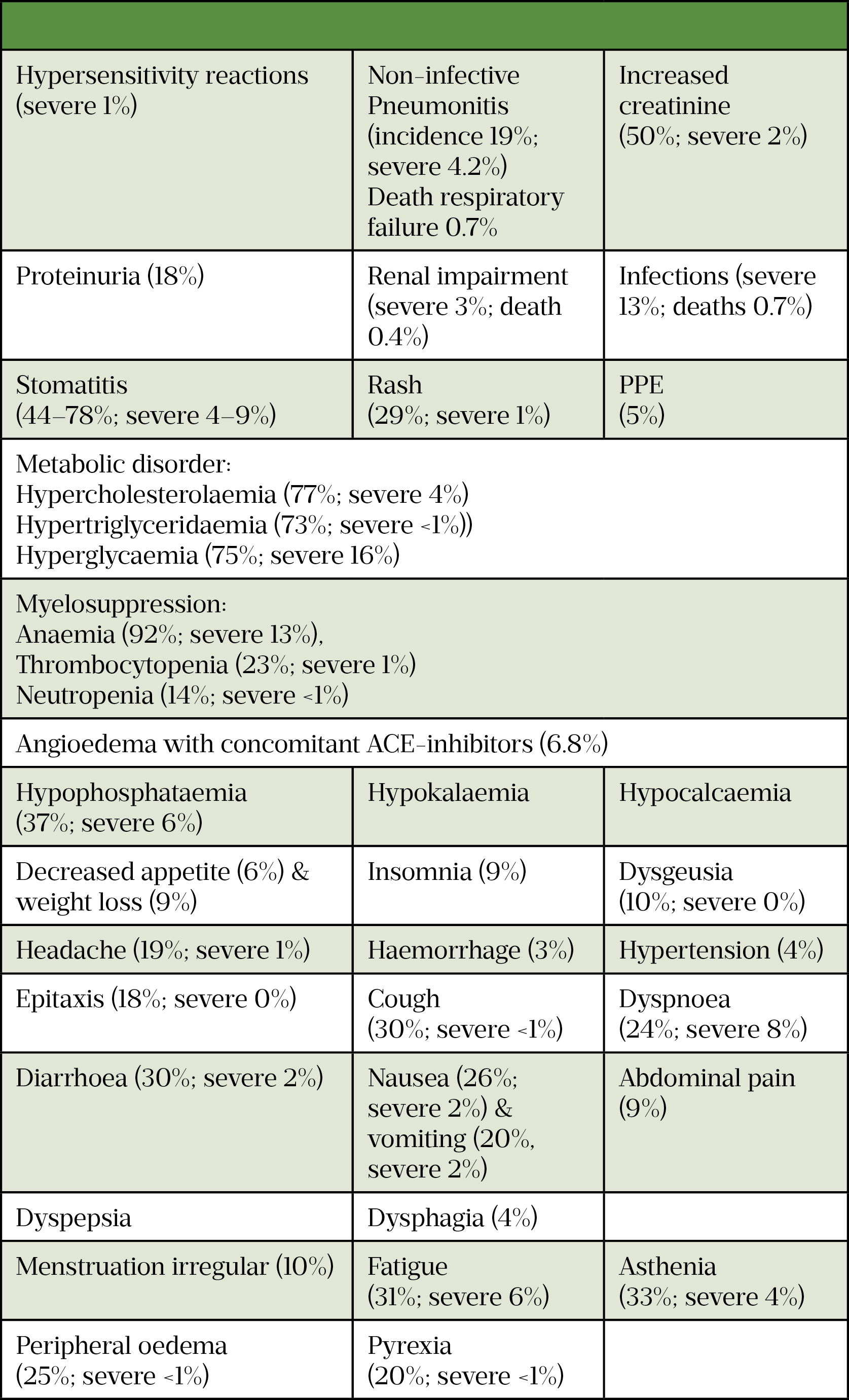
Everolimus can be used in combination with lenvatinib and has a lot of overlapping AEs with VEGF TKIs (see Table 8)[112–114]. Everolimus has immunosuppressive properties and may predispose patients to increased risk of infection, but can also mean vaccinations may be less effective during treatment and live vaccines should be avoided during treatment[113].
The role of the pharmacy team
The entire pharmacy team has an important role in the public health message to reduce smoking, reduce obesity and reduce blood pressure. This is imperative to reducing the morbidity and mortality of many diseases, including RC. Identification of red flags for cancer is vital to earlier diagnosis of all cancers.
Drug treatments for metastatic RCC have toxicities that require monitoring and there are roles for pharmacists/pharmacy technicians in the medicines management of this cancer. This can include:
- Offering advice on withholding treatments until side effects subside;
- Dose reductions;
- Management plans to reduce the burden of side effects;
- Prescribing medicines for side effects, such as mouthwashes for mucositis or options for management of diarrhoea.
The treatments require monitoring of blood tests as they can cause an array of problems, such as hepatotoxicity, both hypothyroid and hyperthyroidism and electrolyte disturbance (potassium, calcium, magnesium, phosphate). The pharmacy team can be involved in the ordering of blood tests, the direct review of the blood results and any action required should a blood test be out of range.
Pharmacy technicians can also positively influence medicine adherence and drug counselling, while a specialist pharmacist may be involved in dosing, toxicity management and advising on pharmaceutical aspects, such as drug–drug interactions, drug–food interactions, monitoring parameters and stopping treatment prior to surgery.
Treatment of RCC is a prime example of an area where an expanded role for the pharmacy team, and particularly independent prescribing pharmacist roles, can add significant value to the wider healthcare team.
Summary
Our understanding of biological pathways and novel targeted therapies has led to improved survival outcomes in patients with metastatic disease. The inclusion of ICI-based therapy as first-line treatment of mRCC has improved outcomes for patients with mRCC and is standard of care for most patients.
Useful resources
- 1Kidney cancer statistics. Cancer Research UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer#heading-Zero (accessed Jun 2022).
- 2Cumberbatch MG, Rota M, Catto JWF, et al. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. European Urology. 2016;70:458–66. doi:10.1016/j.eururo.2015.06.042
- 3Tsivian M, Moreira DM, Caso JR, et al. Cigarette Smoking Is Associated With Advanced Renal Cell Carcinoma. JCO. 2011;29:2027–31. doi:10.1200/jco.2010.30.9484
- 4Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int. J. Cancer. 2005;118:728–38. doi:10.1002/ijc.21398
- 5Hidayat K, Du X, Zou S-Y, et al. Blood pressure and kidney cancer risk. Journal of Hypertension. 2017;35:1333–44. doi:10.1097/hjh.0000000000001286
- 6Mandel JS, McLaughlin JK, Schlehofer B, et al. International renal-cell cancer study. IV. Occupation. Int. J. Cancer. 1995;61:601–5. doi:10.1002/ijc.2910610503
- 7Argani P, Laé M, Ballard ET, et al. Translocation Carcinomas of the Kidney After Chemotherapy in Childhood. JCO. 2006;24:1529–34. doi:10.1200/jco.2005.04.4693
- 8Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. European Urology. 2019;75:799–810. doi:10.1016/j.eururo.2019.02.011
- 9Cheungpasitporn W, Thongprayoon C, O’Corragain OA, et al. The risk of kidney cancer in patients with kidney stones: a systematic review and meta-analysis. QJM. 2014;108:205–12. doi:10.1093/qjmed/hcu195
- 10Gordon SC, Moonka D, Brown KA, et al. Risk for Renal Cell Carcinoma in Chronic Hepatitis C Infection. Cancer Epidemiology, Biomarkers & Prevention. 2010;19:1066–73. doi:10.1158/1055-9965.epi-09-1275
- 11Lowrance WT, Ordoñez J, Udaltsova N, et al. CKD and the Risk of Incident Cancer. JASN. 2014;25:2327–34. doi:10.1681/asn.2013060604
- 12Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2019;30:706–20. doi:10.1093/annonc/mdz056
- 13Tran J, Ornstein MC. Clinical Review on the Management of Metastatic Renal Cell Carcinoma. JCO Oncology Practice. 2022;18:187–96. doi:10.1200/op.21.00419
- 14Clinical review: renal cell carcinoma. Clinical Keys. 2021.https://www.clinicalkey.com/#!/content/clinical_overview/67-s2.0-b1f0ac9a-0dd1-47e1-8000-68f28862097d (accessed Jun 2022).
- 15Utilising community pharmacists to support people with cancer. Royal Pharmaceutical Society. 2020.https://www.rpharms.com/recognition/all-our-campaigns/policy-a-z/cancer-support (accessed Jun 2022).
- 16Capitanio U, Montorsi F. Renal cancer. The Lancet. 2016;387:894–906. doi:10.1016/s0140-6736(15)00046-x
- 17Linehan WM, Rathmell WK. Kidney cancer. Urologic Oncology: Seminars and Original Investigations. 2012;30:948–51. doi:10.1016/j.urolonc.2012.08.021
- 18Warren AY, Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies. World J Urol. 2018;36:1913–26. doi:10.1007/s00345-018-2447-8
- 19Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras. 2015;48:166–74. doi:10.1590/0100-3984.2013.1927
- 20Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 8th ed. Oxford, UK: : Wiley Blackwell 2016. https://www.uicc.org/resources/tnm-classification-malignant-tumours-8th-edition (accessed Jun 2022).
- 21Survival Rates for Kidney Cancer. American Cancer Society. https://www.cancer.org/cancer/kidney-cancer/detection-diagnosis-staging/survival-rates.html (accessed Jun 2022).
- 22Linehan WM, Pinto PA, Bratslavsky G, et al. Hereditary kidney cancer. Cancer. 2009;115:2252–61. doi:10.1002/cncr.24230
- 23Moore LE, Nickerson ML, Brennan P, et al. Von Hippel-Lindau (VHL) Inactivation in Sporadic Clear Cell Renal Cancer: Associations with Germline VHL Polymorphisms and Etiologic Risk Factors. PLoS Genet. 2011;7:e1002312. doi:10.1371/journal.pgen.1002312
- 24Haas NB, Nathanson KL. Hereditary Kidney Cancer Syndromes. Advances in Chronic Kidney Disease. 2014;21:81–90. doi:10.1053/j.ackd.2013.10.001
- 25Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. The Lancet. 2003;361:2059–67. doi:10.1016/s0140-6736(03)13643-4
- 26Roberto M, Botticelli A, Panebianco M, et al. Metastatic Renal Cell Carcinoma Management: From Molecular Mechanism to Clinical Practice. Front. Oncol. 2021;11. doi:10.3389/fonc.2021.657639
- 27Tian T, Li X, Zhang J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. IJMS. 2019;20:755. doi:10.3390/ijms20030755
- 28Chertow G, Luyckx V, Marsden P, et al. Brenner and Rector’s The Kidney. 56th ed. Philadelphia, Pennsylvania: : Elsevier 2019. https://www.elsevier.com/books/brenner-and-rectors-the-kidney-2-volume-set/yu/978-0-323-53265-5 (accessed Jun 2022).
- 29Rini BI. Vascular Endothelial Growth Factor–Targeted Therapy in Renal Cell Carcinoma: Current Status and Future Directions. Clinical Cancer Research. 2007;13:1098–106. doi:10.1158/1078-0432.ccr-06-1989
- 30Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–9. doi:10.1038/nature12222
- 31Immune checkpoint inhibitors in cancer: pharmacology and toxicities. Pharmaceutical Journal. 2018. doi:10.1211/pj.2018.20204831
- 32Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 Is Associated with Poor Prognosis in Renal Cell Carcinoma Patients with Long-term Follow-up. Cancer Research. 2006;66:3381–5. doi:10.1158/0008-5472.can-05-4303
- 33Choueiri TK, Fay AP, Gray KP, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Annals of Oncology. 2014;25:2178–84. doi:10.1093/annonc/mdu445
- 34Lal A, Sahu KK, Jindal V, et al. Role of immunotherapy in metastatic renal cell cancer: past, present and future. Ann Transl Med. 2019;7:S349–S349. doi:10.21037/atm.2019.09.95
- 35MacLennan S, Imamura M, Lapitan MC, et al. Systematic Review of Oncological Outcomes Following Surgical Management of Localised Renal Cancer. European Urology. 2012;61:972–93. doi:10.1016/j.eururo.2012.02.039
- 36Staging and Grading of Kidney Cancer. Kidney Cancer UK. 2022.https://www.kcuk.org.uk/kidneycancer/symptoms-and-diagnosis/staging-and-grading-of-kidney-cancer/ (accessed Jun 2022).
- 37Patard J-J, Leray E, Rioux-Leclercq N, et al. Prognostic Value of Histologic Subtypes in Renal Cell Carcinoma: A Multicenter Experience. JCO. 2005;23:2763–71. doi:10.1200/jco.2005.07.055
- 38Sun M, Marconi L, Eisen T, et al. Adjuvant Vascular Endothelial Growth Factor–targeted Therapy in Renal Cell Carcinoma: A Systematic Review and Pooled Analysis. European Urology. 2018;74:611–20. doi:10.1016/j.eururo.2018.05.002
- 39Pembrolizumab for adjuvant treatment of renal cell carcinoma [ID3810]. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/indevelopment/gid-ta10693 (accessed Jun 2022).
- 40Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021;385:683–94. doi:10.1056/nejmoa2106391
- 41Powles T, Albiges L, Bex A, et al. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Annals of Oncology. 2021;32:1511–9. doi:10.1016/j.annonc.2021.09.014
- 42Motzer RJ, Mazumdar M, Bacik J, et al. Survival and Prognostic Stratification of 670 Patients With Advanced Renal Cell Carcinoma. JCO. 1999;17:2530–2530. doi:10.1200/jco.1999.17.8.2530
- 43Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. The Lancet Oncology. 2013;14:141–8. doi:10.1016/s1470-2045(12)70559-4
- 44Heng DYC, Xie W, Regan MM, et al. Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor–Targeted Agents: Results From a Large, Multicenter Study. JCO. 2009;27:5794–9. doi:10.1200/jco.2008.21.4809
- 45Guida A, Le Teuff G, Alves C, et al. Identification of international metastatic renal cell carcinoma database consortium (IMDC) intermediate-risk subgroups in patients with metastatic clear-cell renal cell carcinoma. Oncotarget. 2020;11:4582–92. doi:10.18632/oncotarget.27762
- 46Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. The Lancet Oncology. 2015;16:293–300. doi:10.1016/s1470-2045(14)71222-7
- 47West H (Jack), Jin JO. Performance Status in Patients With Cancer. JAMA Oncol. 2015;1:998. doi:10.1001/jamaoncol.2015.3113
- 48Guidance on the use of temozolomide for the treatment of recurrent malignant glioma (brain cancer) Technology appraisal guidance [TA23]. National Institute for Health and Care Excellence. 2016.https://www.nice.org.uk/guidance/ta23/chapter/Appendix-D-Karnofsky-Performance-Score (accessed Jun 2022).
- 49Coppin C, Porzsolt F, Autenrieth M, et al. Immunotherapy for advanced renal cell cancer. Cochrane Database of Systematic Reviews. 2004. doi:10.1002/14651858.cd001425.pub2
- 50Fyfe GA, Fisher RI, Rosenberg SA, et al. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. JCO. 1996;14:2410–1. doi:10.1200/jco.1996.14.8.2410
- 51Pazopanib for the first-line treatment of advanced renal cell carcinoma. National Institute for Health and Care Excellence. 2013.https://www.nice.org.uk/guidance/ta215 (accessed Jun 2022).
- 52Sunitinib for the first-line treatment of advanced and/or metastatic renal cell carcinoma. National Institute for Health Care Excellence. 2009.https://www.nice.org.uk/guidance/ta169 (accessed Jun 2022).
- 53Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378:1277–90. doi:10.1056/nejmoa1712126
- 54Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long‐term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128:2085–97. doi:10.1002/cncr.34180
- 55Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5:e001079. doi:10.1136/esmoopen-2020-001079
- 56Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Annals of Oncology. 2020;31:1030–9. doi:10.1016/j.annonc.2020.04.010
- 57Motzer RJ, Robbins PB, Powles T, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med. 2020;26:1733–41. doi:10.1038/s41591-020-1044-8
- 58Motzer R, Alekseev B, Rha S-Y, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384:1289–300. doi:10.1056/nejmoa2035716
- 59Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–27. doi:10.1056/nejmoa1816714
- 60Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2021;384:829–41. doi:10.1056/nejmoa2026982
- 61Motzer RJ, Nosov D, Eisen T, et al. Tivozanib Versus Sorafenib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma: Results From a Phase III Trial. JCO. 2013;31:3791–9. doi:10.1200/jco.2012.47.4940
- 62Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. JCO. 2010;28:1061–8. doi:10.1200/jco.2009.23.9764
- 63Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2013;369:722–31. doi:10.1056/nejmoa1303989
- 64Motzer RJ, Hutson TE, Tomczak P, et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. JCO. 2009;27:3584–90. doi:10.1200/jco.2008.20.1293
- 65Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. JCO. 2017;35:591–7. doi:10.1200/jco.2016.70.7398
- 66Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. European Journal of Cancer. 2018;94:115–25. doi:10.1016/j.ejca.2018.02.012
- 67Tivozanib for treating advanced renal cell carcinoma. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/ta512 (accessed Jun 2022).
- 68Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2007;356:115–24. doi:10.1056/nejmoa065044
- 69Avelumab with axitinib for untreated advanced renal cell carcinoma. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ta645 (accessed Jun 2022).
- 70Avelumab (Bavencio). Scottish Medicines Consortium. 2020.https://www.scottishmedicines.org.uk/medicines-advice/avelumab-bavencio-full-smc2248 (accessed Jun 2022).
- 71Pembrolizumab (Keytruda). Scottish Medicines Consortium. 2021.https://www.scottishmedicines.org.uk/medicines-advice/pembrolizumab-keytruda-full-smc2247 (accessed Jun 2022).
- 72Cabozantib (Cabometyx). Scottish Medicines Consortium. 2021.https://www.scottishmedicines.org.uk/medicines-advice/cabozantinib-cabometyx-abb-smc2386 (accessed Jun 2022).
- 73Nivolumab with ipilimumab for untreated advanced renal cell carcinoma. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta581 (accessed Jun 2022).
- 74Nivolumab (Opdivo). Scottish Medicines Consortium. Scottish Medicines Consortium. 2019.https://www.scottishmedicines.org.uk/medicines-advice/nivolumab-opdivo-fullsubmission-smc2153 (accessed Jun 2022).
- 75Cabozantinib for untreated advanced renal cell carcinoma. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/ta542 (accessed Jun 2022).
- 76Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. The Lancet Oncology. 2016;17:917–27. doi:10.1016/s1470-2045(16)30107-3
- 77Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–13. doi:10.1056/nejmoa1510665
- 78Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long‐term follow‐up of the randomized, open‐label, phase 3 CheckMate 025 trial. Cancer. 2020;126:4156–67. doi:10.1002/cncr.33033
- 79Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. The Lancet Oncology. 2015;16:1473–82. doi:10.1016/s1470-2045(15)00290-9
- 80Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. The Lancet Oncology. 2013;14:552–62. doi:10.1016/s1470-2045(13)70093-7
- 81Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma. Cancer. 2010;116:4256–65. doi:10.1002/cncr.25219
- 82Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. The Lancet Oncology. 2020;21:95–104. doi:10.1016/s1470-2045(19)30735-1
- 83YERVOY 5 mg/ml concentrate for solution for infusion. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/4683 (accessed Jun 2022).
- 84KEYTRUDA 25 mg/mL concentrate for solution for infusion. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/2498 (accessed Jun 2022).
- 85OPDIVO 10 mg/mL concentrate for solution for infusion. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/6888 (accessed Jun 2022).
- 86Cabometyx 60 mg Film-coated Tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7631/smpc (accessed Jun 2022).
- 87Fotivda 1340mcg hard capsules. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/8995/smpc (accessed Jun 2022).
- 88Inlyta 5 mg film-coated tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7948/smpc (accessed Jun 2022).
- 89Kisplyx 10 mg hard capsules. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7881/smpc (accessed Jun 2022).
- 90SUTENT 50 mg hard capsules. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7966 (accessed Jun 2022).
- 91Votrient 400 mg film coated tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/572/smpc (accessed Jun 2022).
- 92Jonasch E, Donskov F, Iliopoulos O, et al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease. N Engl J Med. 2021;385:2036–46. doi:10.1056/nejmoa2103425
- 93McDermott DF, Choueiri TK, Bauer TM, et al. 656MO Phase II study of belzutifan (MK-6482), an oral hypoxia-inducible factor 2α (HIF-2α) inhibitor, plus cabozantinib for treatment of advanced clear cell renal cell carcinoma (ccRCC). Annals of Oncology. 2021;32:S681. doi:10.1016/j.annonc.2021.08.052
- 94Sivendran S, Liu Z, Portas LJ Jr, et al. Treatment-related mortality with vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy in patients with advanced solid tumors: A meta-analysis. Cancer Treatment Reviews. 2012;38:919–25. doi:10.1016/j.ctrv.2012.05.001
- 95FOTIVDA® (tivozanib) capsules, for oral use. US Food and Drug Administration. 2021.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212904s000lbl.pdf (accessed Jun 2022).
- 96INLYTA® (axitinib) tablets, for oral administration. US Food and Drug Administration. 2020.https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202324s011lbl.pdf (accessed Jun 2022).
- 97LENVIMA® (lenvatinib) capsules, for oral use. US Food and Drug Administration. 2021.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/206947s021lbl.pdf (accessed Jun 2022).
- 98SUTENT® (sunitinib malate) capsules, oral. US Food and Drug Administration. 2006.https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021968s002s003s004s005s006lbl.pdf (accessed Jun 2022).
- 99VOTRIENT® (pazopanib) tablets, for oral use. US Food and Drug Administration. 2021.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022465s031s032lbl.pdf (accessed Jun 2022).
- 100Qi W-X, Tang L-N, Sun Y-J, et al. Incidence and risk of hemorrhagic events with vascular endothelial growth factor receptor tyrosine-kinase inhibitors: an up-to-date meta-analysis of 27 randomized controlled trials. Annals of Oncology. 2013;24:2943–52. doi:10.1093/annonc/mdt292
- 101Izzedine H, Massard C, Spano JP, et al. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. European Journal of Cancer. 2010;46:439–48. doi:10.1016/j.ejca.2009.11.001
- 102Ghatalia P, Je Y, Mouallem NE, et al. Hepatotoxicity with vascular endothelial growth factor receptor tyrosine kinase inhibitors: A meta-analysis of randomized clinical trials. Critical Reviews in Oncology/Hematology. 2015;93:257–76. doi:10.1016/j.critrevonc.2014.11.006
- 103CABOMETYX® (cabozantinib) tablets, for oral use. US Food and Drug Administration. 2012.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208692s012lbl.pdf (accessed Jun 2022).
- 104Shah DR, Dholakia S, Shah RR. Effect of Tyrosine Kinase Inhibitors on Wound Healing and Tissue Repair: Implications for Surgery in Cancer Patients. Drug Saf. 2014;37:135–49. doi:10.1007/s40264-014-0139-x
- 105Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2017;28:iv119–42. doi:10.1093/annonc/mdx225
- 106Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. JCO. 2021;39:4073–126. doi:10.1200/jco.21.01440
- 107Bajwa R, Cheema A, Khan T, et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J Clin Med Res. 2019;11:225–36. doi:10.14740/jocmr3750
- 108Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. j. immunotherapy cancer. 2019;7. doi:10.1186/s40425-019-0645-6
- 109Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9:e002435. doi:10.1136/jitc-2021-002435
- 110Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? European Journal of Cancer. 2019;122:72–90. doi:10.1016/j.ejca.2019.07.014
- 111Grünwald V, Voss MH, Rini BI, et al. Axitinib plus immune checkpoint inhibitor: evidence- and expert-based consensus recommendation for treatment optimisation and management of related adverse events. Br J Cancer. 2020;123:898–904. doi:10.1038/s41416-020-0949-9
- 112AFINITOR® (everolimus) tablets, for oral use. US Food and Drug Administration. 2009.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022334s050lbl.pdf (accessed Jun 2022).
- 113Afinitor 10mg tablets. Electronic Medicines Compendium. 2021.https://www.medicines.org.uk/emc/product/6658/smpc (accessed Jun 2022).
- 114TORISEL 30 mg concentrate and diluent for solution for infusion. Electronic Medicines Compendium. 2017.https://www.medicines.org.uk/emc/product/6401 (accessed Jun 2022).