
Life in View / Science Photo Library
What is revalidation?
In 2018, pharmacists and pharmacy technicians join other healthcare professionals in being expected to undergo the revalidation process, demonstrating their continuing competence and fitness to practise. Revalidation aims to demonstrate that continuing professional development (CPD) is an activity that is undertaken regularly by pharmacy professionals, rather than only when records are called[1]
. Key dates and information on the revalidation process can be found in Box 1.
Box 1: Key dates and information
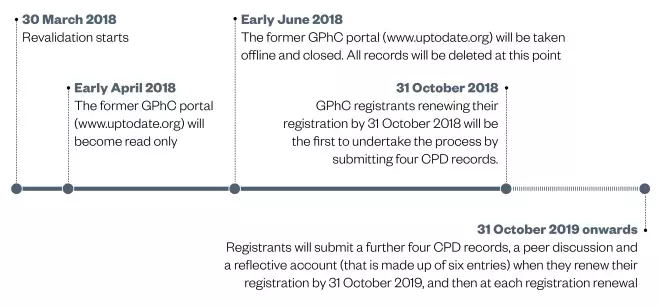
Revalidation came into force on 30 March 2018. The General Pharmaceutical Council (GPhC) will contact registrants about the new process.
Registrants were still expected to meet the existing CPD requirements in the months leading up to revalidation starting in April 2018, but the GPhC will not be considering records related to previous periods — 2018 revalidation will be a fresh start[1],[2]
and the requirements are outlined in this article.
The GPhC has produced a series of personalised revalidation timelines depending on individual registration renewal dates or registration expiry dates, which is available on its website.
Important dates
Evidence and submission requirements
Pharmacy professionals will be required to submit various pieces of work and make declarations to illustrate that they are keeping their skills up to date.
CPD records will be submitted annually, and so are expected to be relevant to the past 12 months of practice. In addition, they should demonstrate the positive impact a learning activity has had on patients, as well as other service users[1],[3]
.
In the first year revalidation records are submitted to the General Pharmaceutical Council (GPhC), the independent regulator for pharmacists, pharmacy technicians and pharmacy premises in Great Britain, four activities will need to be undertaken and recorded. Registrants will need to submit these records by their next renewal deadline, which may fall on or after 31 October 2018[1],[3]
.
In the second year revalidation records are submitted, and for each year following this, registrants will need to undertake and record four CPD records, a peer discussion record and a reflective account record (see Box 2). These will be required on the renewal deadline falling on or after 31 October 2019[1],[3]
.
Box 2: What is a peer discussion and reflective account record?
Peer discussion is a learning and development activity that encourages pharmacy professionals to engage with others in reflection on their learning and practice. Peer discussions aim be open and honest, and aid development. They can be conducted with a trusted and respected individual; however, this does not necessarily need to be a pharmacy professional. They can take place in various formats, including face-to-face discussions or by phone, web chat and video calls[1],[4]
.
Reflective accounts are designed to make pharmacy professionals think about how they meet the GPhC standards in the work they do.
Each year, the GPhC will ask registrants to select one or more of the standards for pharmacy professionals to reflect upon; however, it will explicitly state which standards it expects individuals to reflect upon that year.
Reflective accounts should include a brief summary of the individual’s practice history for the past year, including who the typical users of their service are; a statement of how the individual has met one or more of the standards for pharmacy professionals; and examples to support the statement[1],[4]
.
Types of CPD records
Planned versus unplanned learning
There are two different types of learning that can be recorded as CPD:
Planned learning can be undertaken when the individual decides to develop knowledge or skills in advance of undertaking the learning activity (e.g. attendance at an event or conference, or undertaking a specific activity around a known knowledge gap)[1]
.
Unplanned learning occurs when an event occurs that causes a learning activity to be undertaken or carried out without any prior thought or planning (e.g. through reading a journal, undertaking an activity or task, or a discussion with a colleague owing to an interaction during your normal working day)[1]
.
It is important to note that some learning activity undertaken by registrants is planned, but sometimes beneficial learning may be undertaken or prompted because of specific events during practice, and is therefore unplanned. Both planned and unplanned learning can lead from one to the other[1]
, and further examples of both are included in Table 1 (please note that these examples are not exhaustive).
| Planned learning activities | Unplanned learning activities |
|---|---|
| Review of relevant literature or clinical guidelines | Attendance at workshop, conference or event highlights knowledge gap or a different way of approaching a scenario |
| Attended workshop, conference or event in person or online (e.g. webinar) | Online literature search owing to case encountered that day |
| Reading a summary of product characteristics | Reading a journal article |
| Compiling a background reading list on a topic and reviewing published information services | Consulting additional reference texts, as well as British National Formulary, identifies new information not already known |
| Attending a training session | Researching or attending events in new areas of specialism or interest |
| Shadowing a senior colleague | Discussion of a patient case with a multidisciplinary team results in additional learning activities |
| Finding out about local support services for patients with specific conditions, contacting for advice or to help with signposting patients | Involvement in daily ward round results in search of multiple literature types |
| Undertake a specific CPD activity or online module | Reviewing a colleague’s work prior to them submitting for publication |
| Acting as a peer reviewer for a journal | |
| Planning to read a number of journal articles |
As part of CPD, pharmacy professionals are encouraged to undertake as much learning activity as necessary to support safe and effective practice. However, only four CPD records covering unplanned or unplanned learning need to be submitted.
A minimum of two CPD records must be planned and a maximum of two can be unplanned. Therefore, the four annual CPD records can be split between the two types as long as this requirement is met, they cover activities from the past 12 months, and demonstrate the positive impact the learning activity has had on patients and service users.
The main things to remember are:
- CPD records should include real examples that demonstrate the benefits for patients (while respecting patient confidentiality), rather than hypothetical examples;
- Recording may involve more than one stage (e.g. learning may be undertaken and then be revisited once applied);
- Across the four entries submitted, a wide range of methods should be used to support learning (e.g. conference or event attendance, reading journal articles, literature reviews or national guidelines, completion of specific clinical CPD modules, participation in ward rounds, case discussions, reading a summary of product characteristics, etc.);
- Learning should reflect the context of your practice (e.g. if you have multiple roles or specialisms, records should reflect this).
The GPhC has created a range of different example CPD records for pharmacy professionals working across different sectors to help registrants complete their entries. Further examples of sector-specific planned and unplanned records and CPD form recording templates are available on the RPS revalidation portal.
Online subscribers to The Pharmaceutical Journal and Clinical Pharmacist can also now find planned and unplanned learning CPD form templates at the end of relevant content, including learning, CPD and research articles, so that learning and action points can be recorded easily. Copies of these forms can be emailed directly to users for their records.
What detail should be included in the CPD record?
The GPhC has outlined the details that should be included for each planned and unplanned CPD record entry[1],[5]
. The following can be used as a checklist when creating your records:
Planned CPD learning
Each record should include a description and details of:
- What you want to learn;
- The relevance of the learning to your practice;
- How the learning will affect the people using your services;
- The options or activities you have selected to carry out;
- How you have applied the learning;
- How the learning (once you have applied it) has benefited the people using your services, as illustrated with an example.
Unplanned CPD learning
Each record should include a description and details of:
- The activity you took part in that enabled new learning;
- What you have learned;
- How you have applied the learning;
- How the learning (once you have applied it) has benefited the people using your services, as illustrated with an example.
What happens once CPD records are submitted?
The GPhC will review around 2.5% of the records submitted from the register each year. Some of these will be chosen at random, but others will be specifically targeted (e.g. if records have been submitted late without good reason)[1],[4]
.
If your submission is going to be reviewed, the GPhC will notify you and let you know when you will hear the results. If you meet the criteria outlined above and the records submitted are in order, you usually will not be reviewed again for the next two years[1],[4]
.
If you have not submitted your records to GPhC, without explaining why, they will start a remediation process, which will provide a further opportunity for you to submit your CPD.
How the Royal Pharmaceutical Society is supporting members with revalidation
A dedicated revalidation support hub, which also provides more information on the various support services offered, is available on the Royal Pharmaceutical Society (RPS) website.
- RPS MyCPD app — supported by Pharmaceutical Journal Publications.
- Available for iOS devices via the App Store and Android devices via Google Play
- For more information about how to use the new app, see the accompanying article ‘How to use the new ‘RPS MyCPD’ app for pharmacy revalidation’
- Revalidation roadshows
- The series of revalidation roadshow events aims to help attendees find out everything they need to know about revalidation, how it will affect them, and how the RPS will support them through the process. Revalidation events are open to all pharmacists and pharmacy technicians to attend but booking is essential. Attendance is free-of-charge for RPS members.
- Revalidation support service
- The RPS is providing members with a dedicated Revalidation Support Service, which can be contacted by phone or email:
- 0333 733 2570
- support@rpharms.com
- Revalidation webinar
- A recording of an RPS expert-led webinar is available for members. This webinar aims to provide participants with all the need-to-know information about the new revalidation process and how the RPS provides support.
- Newsletter
- Sign up to the RPS Revalidation newsletter for information regarding any future updates.
-
Revalidation FAQ
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please use the: Planned Learning form
If your learning was spontaneous, please use the: Unplanned Learning form
References
[1] General Pharmaceutical Council. Revalidation framework — January 2018. Available at: https://www.pharmacyregulation.org/sites/default/files/document/gphc_revalidation_framework_january_2018.pdf (accessed April 2018)
[2] General Pharmaceutical Council. Revalidation — FAQs. Available at: https://www.pharmacyregulation.org/revalidation-faqs (accessed April 2018)
[3] Torjesen I. Revalidation: support and implementation. The Pharmaceutical Journal 2018;300(7909):online. doi: 10.1211/PJ.2018.20204267
[4] Torjesen I. Revalidation: the new badge of professionalism. The Pharmaceutical Journal 2017;299(7904):online. doi: 10.1211/PJ.2017.20203414
[5] General Pharmaceutical Council. Revalidation resources for pharmacy professionals. Available at: https://www.pharmacyregulation.org/revalidation-resources-pharmacy-professionals (accessed April 2018)



