This content was published in 2010. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Summary
Around 1% of the UK population has rheumatoid arthritis but it would be hard to find people with the condition in Nigeria, rural China or Indonesia. Environmental and genetic factors contribute to this disparity.
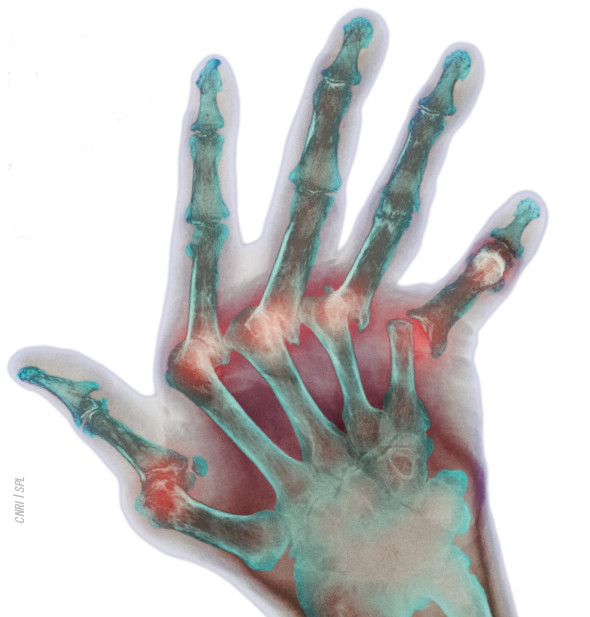
Inflammation of the synovial joints characterises the condition. Early diagnosis (and hence prompt treatment) can prevent progression to joint destruction, disability and systemic effects. However, this is not always easy because a variety of clinical, radiological and serological test results are needed before the condition can be confirmed. Common comorbidities include heart disease, cancer and infection.
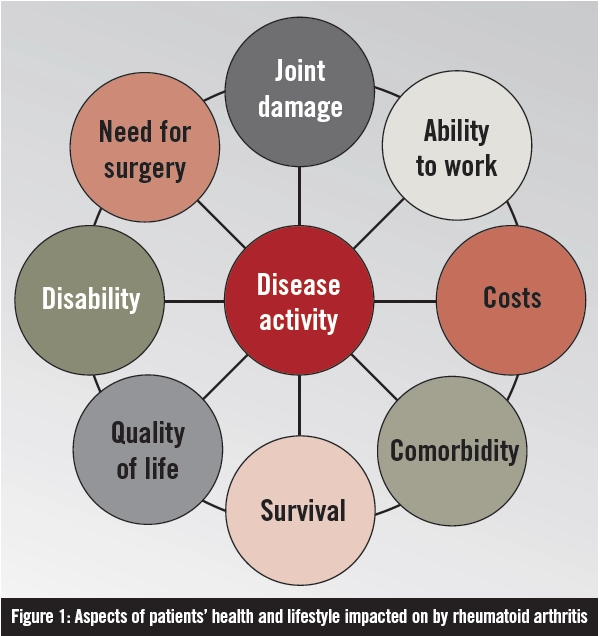
Rheumatoid arthritis (RA) is one of the most common autoimmune diseases. It is a chronic, progressive, systemic inflammatory disorder affecting synovial joints. If left untreated, it leads to joint destruction and, potentially, deformity and disability. As a systemic disease, it is also associated with an increased risk of myocardial infarction and cerebrovascular accident. Many facets of a patient’s life are affected by the condition (see Figure 1), which exerts a considerable financial burden on society both directly (eg, medical care) and indirectly (eg, potential loss of social and financial independence).
Epidemiology
The most reliable estimates of the incidence and prevalence of RA are those derived from population-based studies. Many of these have been undertaken in geographically and ethnically diverse populations and have shown variations in the incidence and prevalence of RA, highlighting the dynamic nature of RA epidemiology.
The annual incidence of RA in the UK is approximately three cases per 10,000 population. However, several studies have shown a decrease in the incidence of RA, particularly in women, over the past few decades.1,2 The initial fall was attributed to a protective effect of the oral contraceptive pill (see later). It is also possible that the decline in incidence is a result of RA becoming less severe and therefore less likely to be diagnosed. There has also been a shift towards an increased age of onset, which is potentially related to the so-called “birth cohort effect”, ie, women born in a particular period might carry a high risk of RA throughout their lives and women from later generations might have a lower risk.
Although the condition affects all populations to some extent, it is more prevalent in some groups than others. For example, in some Native American groups, the prevalence is 5–6%, whereas one large study in Nigeria failed to identify a single case; RA is also rare in rural China and Indonesia. The prevalence in the UK is approximately 1%. There is some evidence to suggest a possible gradient in prevalence going from south to north (eg, in Finland the prevalence of RA in men is 0.6% and in France it is 0.32%). This difference may be explained by genetic or environmental factors.
Genetic causes
It has long been established that RA tends to run in families. First-degree relatives of those with RA are two to 10 times more likely to have the condition than the general population. Genetic clustering in families has been confirmed through observations in twin studies, where concordance among monozygotic twins is approximately 15%, which is up to five times greater than that in dizygotic twins.
Variations in the alleles in the major histocompatibility complex (MHC) on chromosome 6 contribute to the risk of developing almost all of the autoimmune diseases. The MHC locus, otherwise known as the HLA (human leukocyte antigen) region, is involved in the presentation of antigen to T “helper” cells. Studies have shown that many people with RA possess particular HLA-DR alleles (eg, HLA-DR1, HLA-DR4 and HLA-DR10) that share a common sequence in the third hypervariable region of their DRB1 chains. Individuals who are homozygous for the shared epitope have a substantially higher risk of developing RA than those who are heterozygous. However, the contribution of HLA-DRB1 status to the heritability of RA is approximately one third, indicating that there are non-HLA associations. These potentially include the PTPN22 gene on chromosome 1, which encodes for proteins involved in T cell and B cell activation.3,4
Environmental causes
That RA concordance in monozygotic twins is only 15% suggests other non-genetic factors (eg, environmental) contribute to the causation of RA.
Hormones
RA is more common in women than in men, suggesting that female hormones might play a role in its development. RA rarely develops before the first menstrual cycle. The incidence of RA in women who have taken the oral contraceptive pill at some time is around half of that in women who have never taken it. This could be an effect of the pill itself or of delaying pregnancy. Onset of RA during pregnancy is rare, but there is a higher frequency of RA development post-partum, potentially related to breastfeeding (especially after the first pregnancy), because prolactin is proinflammatory.
Infection
Infections have been widely suspected of playing a role in the development of RA, although a definite association is lacking. Bacteria such as Mycobacteria spp, Streptococcus spp and Mycoplasma spp, or an abnormal immunological cross-reactivity between bacterial antigens (eg, from Escherichia coli) and a patient’s cells, have all been suggested as candidates, as has Epstein-Barr virus.
The role of parvovirus in propagating chronic arthritis has been debated for many years. Parvovirus can cause a self-limiting symmetrical inflammatory arthropathy, but progression to chronic RA is rare.
Smoking
Evidence linking smoking and RA is growing. For example, data now indicate that postmenopausal women who currently smoke, or who have stopped smoking for 10 years or less, have a small increased risk of developing RA compared with those who have stopped smoking for more than 10 years. Duration of smoking, rather than intensity, was found to be a risk factor in a study of female health professionals. Current smoking has also been found to be a risk factor for RA, particularly in men who are seropositive (ie, test positive for C-reactive protein and have a high erythrocyte sedimentation rate).
Other
Studies of other environmental factors such as diet and caffeine and alcohol consumption have been inconsistent in their conclusions.
Pathogenesis
RA can affect any joint where cartilage overlies bone and which has a joint cavity lined by synovial membrane that contains synovial fluid. If untreated, RA usually leads to articular destruction and functional disability (Figure 2).
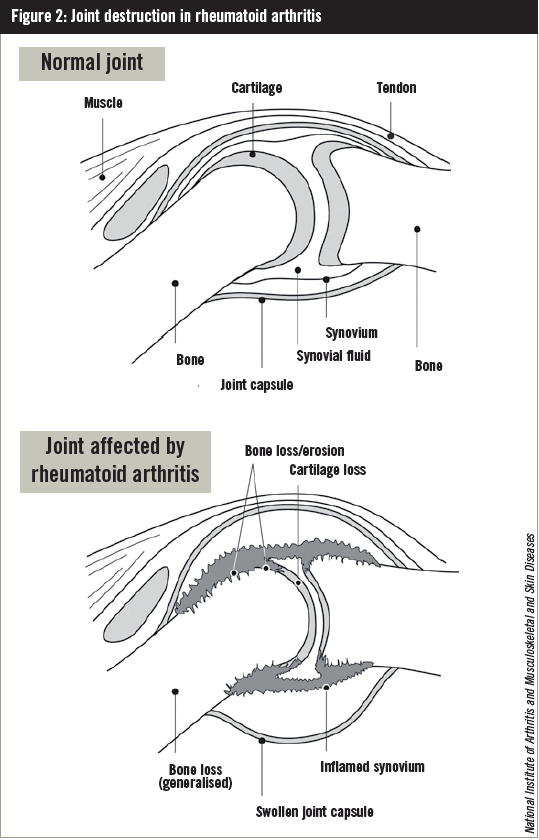
In contrast to many tissue-specific autoimmune diseases, the injury is not caused directly by antigenspecific antibodies (ie, B cells) or T cells but results from the active remodelling processes in the synovium that occur in response to an inflammatory attack. At least three components contribute to joint destruction:
- Transformation of the synovium into a proliferative, tissue-invasive pannus
- Generation of osteoclasts that lead to local resorption of bone
- Attack by cytokines on cartilage cell function
Although synovial inflammation is clinically prominent, RA is systemic at all stages. The most characteristic autoantibodies, rheumatoid factor (RF) and antibodies against citrullinated peptides (anti-CCP), are directed at common antigens expressed widely outside of the joint. Their presence can precede synovial inflammation by decades. Systemic complications manifest as rheumatoid nodules, rheumatoid vasculitis or interstitial lung disease.
Both B cells and T cells have been found in the joints of patients with RA. Role of B cells B cells contribute to the components of joint destruction by stimulating:
- The production of proinflammatory cytokines, such as interleukin (IL)-6, IL-1 and tumour necrosis factor (TNF), which mediate osteoclastogenesis
- The production of autoantibodies, such as RF and anti-CCP, which potentially activate the complement system to stimulate an immune response and also bind to and activate macrophages in the synovium. Macrophages activated by immune complexes produce proinflammatory cytokines that perpetuate inflammation
Role of T cells
T cells potentially activate B cells, resulting in pathogenic antibody production (see above) and also activate macrophages and fibroblasts, resulting in tissue destruction. It remains uncertain as to whether one of these mechanisms, or a combined effect, contributes most to the development of RA.5
Diagnosis
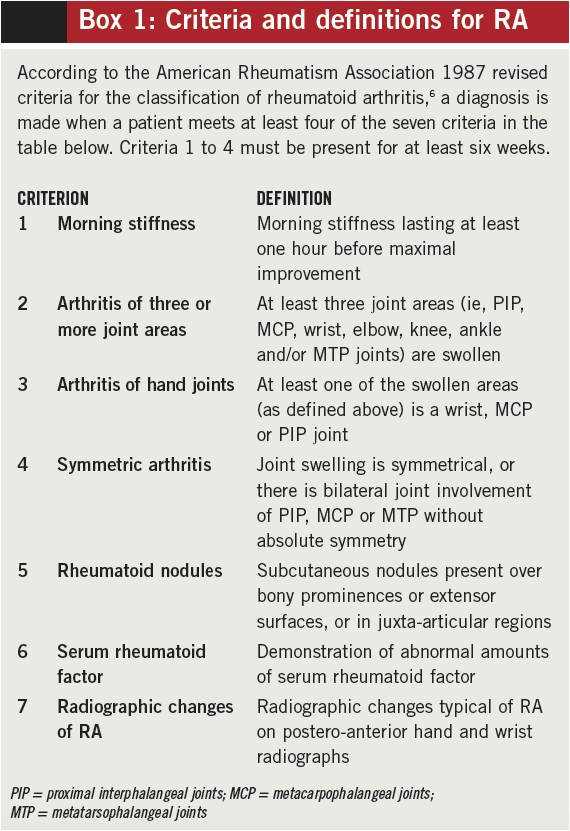
There is no single clinical, radiological or serological test that enables a diagnosis of RA to be made with certainty. As with other rheumatological conditions, diagnosis depends upon the aggregation of characteristic symptoms, signs, laboratory results and radiological findings. In 1987, the American College of Rheumatology established classification criteria (Box 1) to distinguish RA from other established rheumatic disorders.6 These criteria were developed using patients with established RA and therefore have little value in classifying patients with recent onset polyarthritis (ie, they require features to have been present for at least six weeks and therefore cannot be used before that time).
Rapid therapeutic intervention can prevent joint destruction and so early diagnosis is important. In practice, however, early inflammatory arthritis is often undifferentiated and might develop into established rheumatoid arthritis or into another arthropathy, might resolve spontaneously, or might remain undifferentiated.
In an attempt to address this issue, the European League Against Rheumatism (EULAR) has published recommendations for the management of early arthritis. In addition, EULAR and the ACR have jointly proposed new classification criteria for early arthritis.7
Comorbidities
A patient with RA has an average of 1.6 comorbid conditions; this number increases with a patient’s age. Comorbidities add to the complexity of care and make diagnosis and treatment decisions more challenging.
Cardiovascular disease
There is evidence that RA significantly increases the risk of ischaemic heart disease —those with RA have a 3.17-fold higher risk of having an in-hospital myocardial infarction (MI) and a sixfold higher risk of having a silent MI than those without RA. Recent research suggests that RA patients also have an increased risk of heart failure. The increased prevalence of cardiovascular disease is most likely due to atherosclerosis resulting from inflammation,8,9 as well as the presence of other risk factors such as hypertension, dyslipidaemia and smoking.
Cancer
After cardiovascular disease, cancer is the second most common cause of mortality in RA patients. However, the increased risk seems only to relate to a few specific types of cancer, ie, lymphoma, lung cancer and skin cancer.
The increased prevalence of smoking among those with RA contributes, at least in part, to the increase in lung cancer. Since skin cancer is relatively common and often misdiagnosed, it is difficult to determine the effect of RA on its development, although some researchers have found an association between treatment with biologic therapies and an increased risk of developing non-melanoma skin cancer and melanoma.9,10
Other
RA is associated with an increased risk of contracting bacterial, fungal, viral and opportunistic infections, including tuberculosis. This is further increased by the use of immunosuppressant therapies such as disease-modifying antirheumatic drugs, biologics and corticosteroids.9,11 A recent study has shown the incidence of infection to be more than two and a half times that of the non-RA population.
References
- Pederson JK, Kjaer NK, Svendsen AJ, et al. Incidence of rheumatoid arthritis from 1995 to 2001: Impact of ascertainment from multiple sources. Rheumatology International 2009;29:411–15.
- Silman AJ. The changing face of rheumatoid arthritis; why the decline in incidence? Arthritis & Rheumatism 2002;43:1221–30.
- Silman AJ, MacGregor AJ, Thomson W, et al. Twin concordance rates for rheumatoid arthritis: results from a nationwide study. British Journal of Rheumatology 1993;32:835–43.
- Clarke A, Vyse TJ. Genetics of rheumatic disease. Arthritis Research and Therapy 2009;11:248–56.
- Klareskog L, Catrina A, Paget S. Rheumatoid arthritis. Lancet 2009;373:659.
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism 1988;31:305–456.
- Combe B, Landewé R, Lukas C, et al. EULAR recommendations for the management of early arthritis: Report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the Rheumatic Diseases 2007;66:3445.
- Maradit-Kremers H, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis & Rheumatology 2005;52:722–32.
- Mikuls TR. Co-morbidity in rheumatoid arthritis. Best Practice and Research in Clinical Rheumatology 2003;17:729–52.
- Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Research & Therapy 2008;10:R45.
- Doran M, Crowson C, Pond G, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis & Rheumatology 2002;46:2287–93.
You might also be interested in…
Palliative care: end-of-life medicines management
