This content was published in 2009. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Summary
Skin and soft tissue infections (SSTIs) encompass a broad range of infections with a variety of risk factors and causes. Careful assessment of risk factors, severity markers and co-morbidities will inform the most appropriate therapy.
Key clinical decisions include route of administration of therapy, switching from IV to oral therapy, adjunctive measures and suitability for outpatient management. Outpatient parenteral therapy is a viable option for ambulant patients with moderate SSTI requiring IV therapy and without risk factors for severe disease or unstable co-morbidities.
Skin and soft tissue infections (SSTIs) comprise an important and diverse group of anatomically and aetiologically distinct infections. In UK hospitals, 3–4% of patients receive treatment for SSTI. Of these, 47% receive intravenous (IV) therapy, accounting for 16% of all IV antibiotic-treated patients.1 Infections of the skin and subcutaneous tissues account for around 176 admission per 100,000 of the UK population.2
Since the anatomical site, severity, associated comorbidity and aetiology vary, the clinical team managing patients in hospital is likely to include a variety of healthcare professionals in both medical and surgical specialties. This review focuses on important bacterial SSTIs seen in UK hospital practice.
In terms of clinical features and classification, SSTIs may be defined by their involvement of deep structures, by associated risk factors and by their microbiology (see Box 1).

Superficial SSTIs
For people who develop superficial SSTIs, the causative organisms are usually Staphylococcus aureus and Streptococcus pyogenes.
Impetigo is a superficial SSTI rarely associated with systemic upset or extensive skin involvement and more commonly seen in children and young adults. Discrete, multiple lesions usually occur on the face or extremities that are either vesicular-purulent bullous or papular in appearance. Yellow or brown crusting is characteristic. Occasionally, secondary cellulitis can occur.
Folliculitis, furuncles and carbuncles comprise a range of superficial infections involving hair follicles. Folliculitis consists of superficial epidermal inflammation around the follicles; furuncles are small abscesses which may coalesce to form larger carbuncles, usually on the neck.
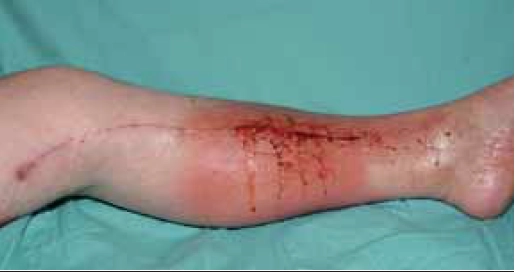
Cellulitis and erysipelas are pathologically distinct dermal infections comprising the most common SSTIs that require admission to hospital and IV antibiotic therapy. Both are diffuse, spreading, superficial infections without underlying suppurative foci in muscle or fascia and without associated necrosis.
Characterised by heat, erythema, induration and localised tenderness, there may also be an “orange skin” appearance, due to superficial oedema surrounding hair follicles which remain tethered to underlying dermis. Blisters or bullae may also occur (Figure 1).
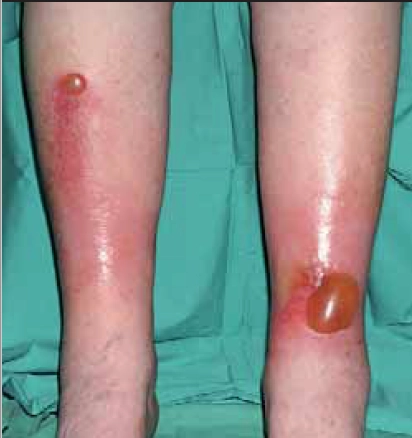
Erysipelas involves the upper dermis and is raised above surrounding skin with a well demarcated edge (Figure 2).
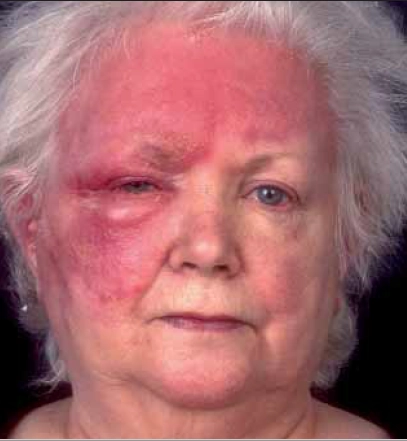
Cellulitis involves deeper dermis and subcutaneous fat, is not raised and is without a well demarcated edge (Figure 3).
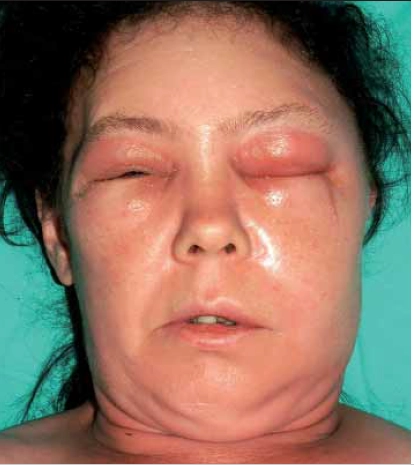
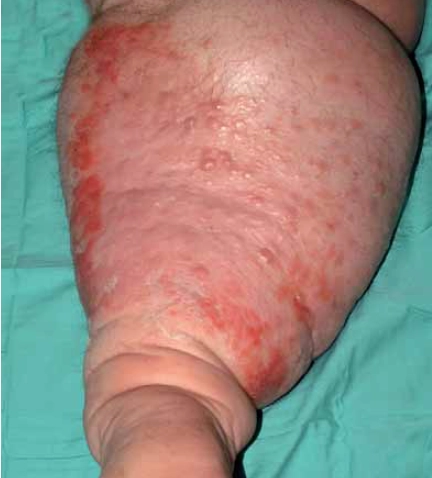
Each may be accompanied by a systemic inflammatory response and regional lymphadenopathy is common. Infection occurs following a minor skin breach, for example an insect bite (more common in the summer months). It may also complicate Tinea pedis or paronychia. Risk of infection is increased in immunocompromised patients, following trauma or surgery, in those with diabetes mellitus or lymphoedema, and in the morbidly obese (Figure 4).
Necrotising SSTIs
Necrotising infection of the skin and soft tissue is severe and life-threatening, with a systemic inflammatory response, involvement of deep tissues, including underlying fascia or muscle, and associated tissue destruction.
Necrotising infections can be distinguished from more superficial infections by the presence of a combination of the following clinical signs: severe, constant pain; blistering and bruising; oedema beyond the margin of the erythema; localised skin anaesthesia; gas in the tissues; systemic inflammatory response and multi-organ failure; and rapidly evolving and spreading infection.
Necrotising fasciitis involves the tissues deep to the dermis and superficial to the muscle. Infection moves along these planes, extending well beyond the superficial signs of infection, and usually occurs as a direct consequence of more superficial infection.
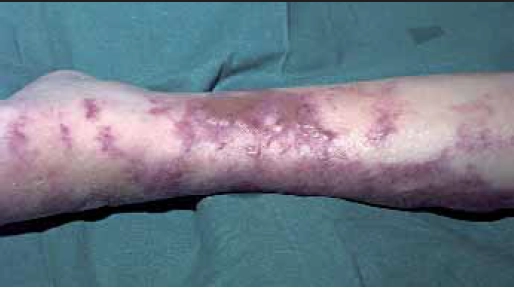
Underlying tissues often feel “wooden” and there may be a dusky discoloration to the skin (Figures 5a and 5b).
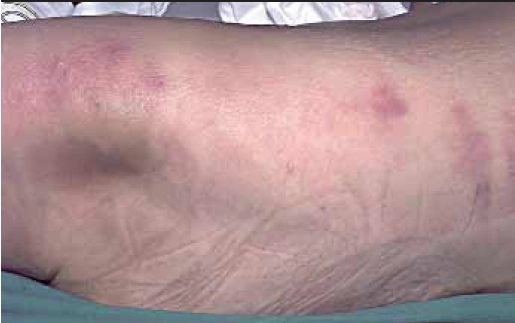
Myositis involves muscle and two distinct groups are recognised: anaerobic streptococcal myositis, usually occurring following surgery or open trauma and involving muscles and fascial planes; and pyomyositis, which is pus within an individual muscle group, usually presenting with localised pain, muscle spasm and fever.
Synergistic necrotising cellulitis is a necrotising soft tissue infection involving muscle groups, in addition to superficial skin and fascia (Figure 6).
Fournier gangrene involves the perineum and genitalia, usually in patients with underlying disease, particularly diabetes mellitus. Onset is usually sudden but can be insidious. An initial superficial focus of infection becomes necrotic and spreads to deep tissues and along fascial planes.
Clostridial myonecrosis (“gas gangrene”) is characterised by severe localised pain, systemic inflammatory response and rapidly evolving skin changes within 24 hours of trauma. Affected areas become tense, fluid-filled blisters develop and gas is visible on plain radiographs.
Spontaneous gangrene can complicate malignancy and neutropenia, is usually blood-borne from a colonic focus and occurs in the absence of trauma.
Microbiology and associated risk factors
Irrespective of site or severity, SSTIs are predominantly caused by aerobic gram-positive cocci, in particular the beta-haemolytic streptococci (notably S pyogenes) and S aureus.3 Other micro-organisms are variably implicated depending on the nature of the SSTI and whether it is healthcare-associated or community-acquired.
Surgical site infection usually occurs more than 48 hours after an incision and is characterised by localised wound-related erythema, heat, induration and purulent discharge.
Involvement of deep structures should always be considered and management depends on the surgical site. In hospitals, S aureus dominates as a cause of surgical-site infection4 (Figure 7), with variable rates of meticillin resistance (see accompanying article, p23).
Animal or human bites can result in infection, and the depth and site of the bite is critical. Hand injuries are common so attention should be paid to potential tendon involvement and the maintenance of function. Therapy is often pre-emptive in view of the high risks of loss of function. Infections are polymicrobial, reflecting oral flora: S aureus, aerobic and anaerobic streptococci, clostridial species, fusobacteria and gram-negative bacteria. With animal bites Pasteurella spp and capnocytophaga are also important.
Water exposure refers to water-related trauma (eg, coral or rock laceration) or contamination with water of an open wound or sore. Both fresh and salinated water harbour micro-organisms and individuals are at potential risk of SSTI following such exposure. Vibrio vulnificus and Aeromonas hydrophilia are frequently responsible.
In hospitals some hydrophilic organisms such as pseudomonas and stenotrophomonas can also cause SSTIs, particularly in compromised, post-operative patients. Mycobacterium marinum infection (or “fish tank granuloma”) most frequently occurs following a laceration incurred when cleaning tropical fish tanks. Systemic infection is unusual.
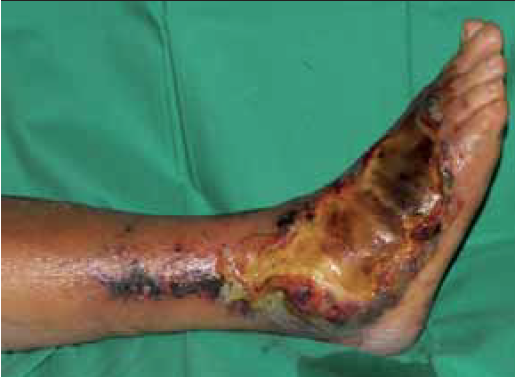
Parenteral drug users are an at-risk group for SSTIs. The full range of infections — ranging from simple injection-site abscesses to necrotising infections — can be seen in inner-city hospitals and clinics. Concomitant blood-stream infection and venous thromboembolism is not uncommon (Figure 8).
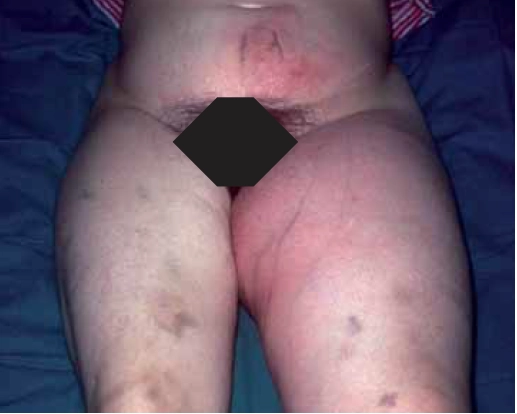
Individuals are at risk through translocation of commensal skin organisms into the blood stream directly, by use of contaminated heroin (usually with heat-resistant organisms), or via contamination during drug preparation. Gram-positive organisms, particularly S aureus and betahaemolytic streptococci, are usually implicated. Clostridial species, particularly C perfringens and C novyi, can cause devastating, rapidly progressive infections associated with marked leucocytosis and systemic inflammatory response.
Immunocompromised patients may develop SSTIs, with S aureus and S pyogenes as the predominant organisms in this diverse patient group. Gram-negative organisms, including Pseudomonas aeroginosa, should be considered in the context of neutropenia and line-related SSTI.
Fungal infections (eg, with Fusarium, Aspergillus or Sporothrix spp) are less frequently seen, but may occur in association with neutropenia, organ transplant or longterm immunosuppressive therapy. Their presentation is variable but may consist of papullar, erythematous or purple eruptions with lymphatic spread or erythema and skin ulceration. Fungal infections can occur either as a primary complication or in the context of disseminated infection with multi-organ involvement.
Mycobacterial infections are uncommon and can be indistinguishable from fungal infections but should be considered in the same population.
Travel-related or tropical skin infections are not uncommon in migrants or people returning from abroad.
In addition to the usual bacterial species, infection with a variety of endemic mycoses, mycobacteria (eg, M tuberculosis and M ulcerans) and parasites (eg, Leishmania spp) are possible, depending on the source of exposure.
Investigation and management of SSTIs
Severity of SSTI can be determined by several clinical factors: extent and intensity of inflammation; distribution and depth of infection; presence of systemic inflammatory response; and significant co-morbidities. These markers will help clinicians decide a patient’s suitability for treatment in the community or hospital and whether parenteral or oral therapy is appropriate (Figure 9). Consideration of these factors will direct the antimicrobial therapy.
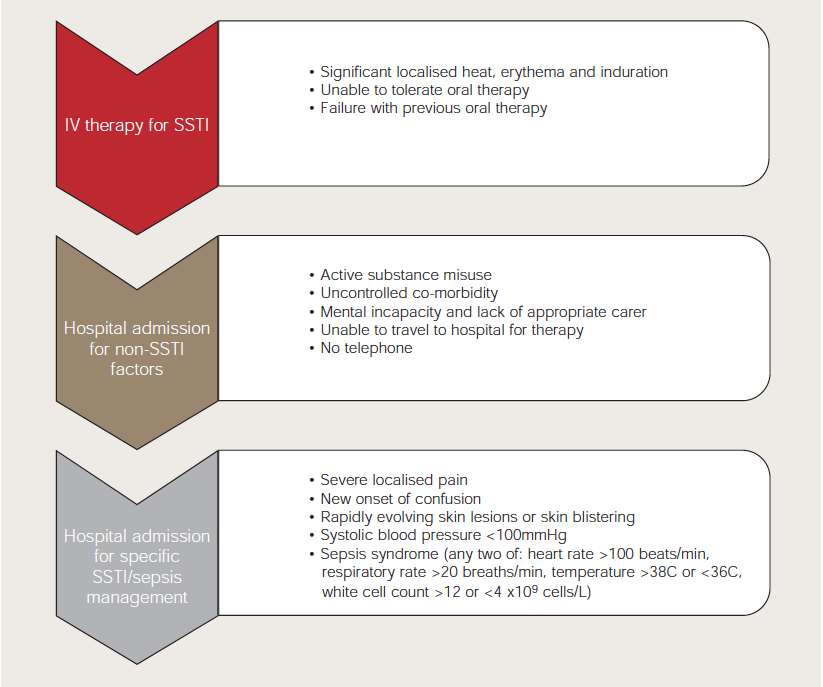
Folliculitis and furuncles are usually treated by GPs using topical antibiotic treatment, or short-course oral therapy if the infection fails to respond. Imprudent and prolonged topical therapy is not advised due to the risk of promotion of bacterial resistance. More extensive SSTIs — such as localised and limited cellulitis with no systemic inflammatory response or significant co-morbidities, and with no compounding microbiological risk factors — can be safely managed with oral antibiotic therapy in the community and without hospital admission.
If signs of localised inflammation persist or worsen then parenteral therapy either as an outpatient or inpatient is indicated. Patients with cellulitis or erysipelas, with significant heat, erythema and induration, generally require parenteral therapy. Deep-seated and necrotising infections always require hospital admission for parenteral therapy and surgical intervention. Patients with postoperative wound infections, particularly following joint or abdominal (or perineal) surgery, should also be admitted for surgical assessment.
For all patients treated with parenteral therapy or managed in the hospital environment, attempts should be made to establish a microbiological diagnosis. In almost all patients with cellulitis or erysipelas there is no exudate and therapy is empiric. Swabs in these circumstances may give misleading results, although evidence of meticillinresistant S aureus (MRSA) carriage does influence empirical choice. Blood cultures are rarely positive. Nevertheless, they are important prognostically and in directing route and duration of therapy, and, therefore, form part of the severity assessment.
Patients with a discharging wound should have a swab performed. However, results should be interpreted with some caution as they may reflect commensal flora. Ideal specimens are obtained aseptically in theatre from the inflamed tissues. In the case of severe SSTI, it is not appropriate to delay antibiotics in order to obtain microbiological specimens and, therefore, specimens are usually obtained after starting parenteral therapy.
Other useful investigations include full blood count, renal function and C reactive protein (CRP). The latter is often normal in patients with cellulitis and erysipelas but is raised in those with severe infections where there is a systemic inflammatory response. CRP can also be useful in the monitoring of more severe infections, particularly when the microbial cause is uncertain. Plain radiographs are useful to assess for subcutaneous gas and soft-tissue oedema. Radiographs are less useful in assessing for acute bony involvement. Computerised tomography and ultrasound examination are used to assess for deep-tissue, bone and joint involvement and for abscess formation. In rapidly progressive necrotising infections, surgical management may be both diagnostic and therapeutic — exploring and debriding fascial planes and muscle compartments to determine the extent and severity of the infection.
Antibiotic therapy
Antibiotic choices for SSTI vary between specialties and institutions, reflecting differing patient populations, anatomical site, resistance patterns, MRSA risk (see accompanying article, p23) and local policy.
Published guidance is deliberately non-prescriptive with respect to antibiotic choice, in part reflecting these complexities, but also because SSTI clinical trials typically exclude the most severely ill patients and are powered only to show non-inferiority between agents.5,6
For patients admitted to hospital requiring IV treatment — and where fully sensitive organisms are isolated or suspected and there is no history of penicillin allergy — narrow-spectrum beta-lactam antibiotics such as benzylpenicillin (for beta-haemolytic streptococci) and flucloxacillin (for both beta-haemolytic streptococci and staphylococci) remain the antibiotics of choice. It is the author’s practice to use flucloxacillin monotherapy as first-line treatment for non-allergic patients unless MRSA or polymicrobial infection is suspected following assessment (see Box 1).
When oral therapy is indicated flucloxacillin is appropriate, and for the beta-lactam-sensitive patient erythromycin or clarithromycin, clindamycin, or doxycycline (except during pregnancy or lactation and for children) are efficacious. For patients with beta-lactam sensitivity requiring IV therapy, vancomycin or clindamycin is usually selected.
For adults with severe SSTIs requiring IV therapy, it is the author’s practice, following administration of an initial IV dose, to use a continuous infusion of either flucloxacillin (eg, 12g/24h) or vancomycin (eg, 2g/24h), to provide the maximum time for the antibiotic to be above the minimum inhibitory concentration for the suspected organism. Therapeutic drug monitoring should be performed for patients receiving vancomycin, aiming for a random-level concentration of 10–15mg/L, with higher concentrations appropriate for patients with MRSA bacteraemia.
For patients with necrotising or rapidly progressive infections, IV clindamycin at a dose of 900mg eighthourly is added to enhance cover against toxigenic S pyogenes. Clindamycin reduces the production of streptococcal toxic shock protein by its action on bacterial mitochondria. It is also active when beta-lactams are rendered ineffective, which occurs during the static growth phase of streptococci when penicillin binding protein production is halted.
If polymicrobial infection is suspected the spectrum of antibiotic cover should be expanded. Typically, for infected bites co-amoxiclav (IV or oral) is appropriate. Doxycyline is a suitable oral alternative if the patient is allergic to beta-lactams. Gentamicin, vancomycin and metronidazole can be considered as alternatives, but specialist advice should be sought and therapy adjusted depending on microbiological results.
Adjunctive measures
All patients with lower-limb SSTI should be assessed for signs of T pedis, which should be treated with topical imidazole antifungal (eg, miconazole) or terbinafine. For severe tinea infections oral terbinafine may be required. Rest and leg elevation are also important in speeding recovery from lower-limb SSTI.
Severe SSTIs should be managed in a highdependency setting with broad antibiotic therapy, fluid resuscitation and appropriate imaging, to delineate the extent and nature of the infection. Frequent clinical review and early surgical review are essential. For patients with necrotising fasciitis, aggressive surgical debridement akin to radical tumour resection, with wide margins of excision of affected tissues, can be life saving — although limb amputation or extensive skin and tissue loss is frequent and mortality high (>60%). Normal human immunoglobulin infusion for 72 hours is used by many infectious diseases physicians in these circumstances in an attempt to neutralise streptococcal toxic shock protein.7
Surgical review should also be sought for SSTIs occurring from a surgical procedure and for all patients with a significant bite or trauma. Careful attention should be paid towards the potential for involvement of deep structures and prosthetic implants.
Outpatient parenteral antibiotic therapy
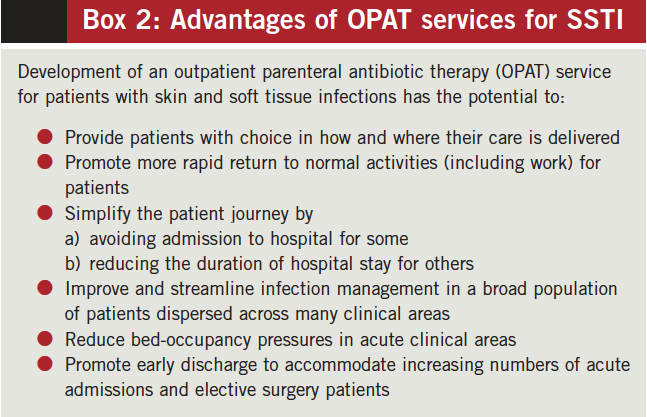
Outpatient parenteral antibiotic therapy (OPAT) is a means to facilitate safe and effective delivery of parenteral antimicrobial therapy, in a non-inpatient setting, to patients for whom IV treatment is the most appropriate choice (Box 2). For greatest efficiency, OPAT should be available soon after presentation to avoid admission or plan early discharge.
Different models exist: an “integrated healthcare at home service” can manage SSTIs in conjunction with other non-infectious conditions, including deep-vein thrombosis, and takes place via acute admissions unit; a “comprehensive infection service” utilises infection specialists (usually infectious diseases physicians), overseeing the management of a range of infectious conditions in the hospital outpatient setting.8 In the US, OPAT is often delivered in the community, usually by a contracted private healthcare provider in an infusion centre, overseen by an infection specialist.9 There are advantages and disadvantages to each model and they can be adapted to local economics and strategies.
Contraindications to OPAT include uncontrolled local infection or sepsis syndrome, unstable co-morbidities, unsuitability for self-care or lack of appropriate home support (Figure 9). When infection is non-severe and rapidly improving, and when there is an appropriate oral agent and swallowing and absorption are not compromised, OPAT is not appropriate — unless the infecting organism is resistant to the available oral therapies. An OPAT antibiotic should be appropriate for the suspected infecting organism, have proven efficacy in SSTI and have a predictable and non-life-threatening toxicity profile. Because OPAT is usually of short duration, once-daily treatment is preferred, combined with clinical review to ensure timely consideration of simplification to oral therapy. For those without true penicillin allergy and at low risk of MRSA, IV or IM ceftriaxone is used.8
Ceftriaxone is bactericidal against both streptococci and meticillin-sensitive staphylococci, with activity against community-acquired enterobacteriaceae. The drug’s half-life is seven to eight hours and serum concentrations suitable to clear most meticillin-sensitive S aureus and streptococcal species are maintained throughout most of the once-daily dosing interval.10
Ertapenem has broad cover (with additional sensitivity against anaerobes), its half-life is long (hence once-daily dosing) and it is suitable for polymicrobial infections, particularly infected bites. Ceftriaxone is preferred for OPAT because of its lower cost and long-term experience with its use.
Teicoplanin is highly protein-bound, has a long halflife and has a good tolerability profile with a sound track record in SSTI. It is, therefore, a suitable alternative to ceftriaxone for patients with true beta-lactam allergy.
Intravenous-to-oral antibiotic switch
Switching antibiotics from IV to oral should occur after a significant reduction in heat, erythema and induration, and with resolution of the systemic inflammatory response. The median duration of IV therapy is three to five days and it is unusual for patients to require IV antibiotics for more than 10 days. Oral treatment following IV therapy should be as for initial oral therapy (as above) and continued for a further five to seven days. Guidelines have been developed by some NHS organisations for suitably trained and experienced non-medical prescribers to facilitate rapid, streamlined IV-to-oral switching in OPAT without the need for scheduled medical input.8
Prevention of recurrent SSTI
Lymphoedema, obesity, diabetes and chronic recurrent T pedis predispose individuals to recurrent SSTI. In frequent recurrences, underlying bony involvement should be considered. Patients with lower-limb SSTI should be counselled on suitable footwear and on prevention of tinea recurrence by regular cleaning and drying of the web spaces and early antifungal therapy.
Antibiotic prophylaxis should be considered for patients requiring repeated IV treatment or hospital admission. Because streptococcal species are the most frequently recurring organisms, twice-daily phenoxymethylpenicillin prophylaxis could be considered. Other options include doxycycline, co-trimoxazole and erythromycin. For patients with recurrent, rapidly progressive, severe infections it is the author’s practice to give (with counselling) take-home antibiotics for use at the earliest sign of infection.
ACKNOWLEDGEMENT
The author would like to thank Kirsty Lattka from medical illustration services at Gartnavel General Hospital, Glasgow, for arranging the photographs published.
References
- Seaton RA, Nathwani D, Burton P, et al. Point prevalence survey of antibiotic use in Scottish hospitals utilising the Glasgow Antimicrobial Audit Tool (GAAT). International Journal of Antimicrobial Agents 2007;29:693–9.
- ISD Scotland. Scottish inpatient, day case and outpatient statistics. www.isdscotland.org/isd/4334.html (accessed 9 December 2008).
- Carratalà J, Rosón B, Fernández-Sabé N, et al. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. European Journal of Clinical Microbiology & Infectious Diseases 2003;22:151–7.
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization and extra costs. Infection Control and Hospital Epidemiology 1999;20:725–30.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections. Clinical Infectious Diseases 2005;41:1373–406.
- Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. Journal of Antimicrobial Chemotherapy 2003;52(s1):i3–17.
- Darenberg J, Ihendyane N, Sjölin J, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clinical Infectious Diseases 2003;37:333–40.
- Seaton RA, Bell, E, Gourlay Y, et al. Nurse-led management of uncomplicated cellulitis in the community; evaluation of a protocol incorporating intravenous ceftriaxone. Journal of Antimicrobial Chemotherapy 2005;55:764–7.
- Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clinical Infectious Diseases 2004;38:1651–72.
- Scully BE, Fu KP, Neu HC. Pharmacokinetics of ceftriaxone after intravenous infusion and intramuscular injection. American Journal of Medicine 1984;77:112–6.
You might also be interested in…
Palliative care: end-of-life medicines management
