This content was published in 2007. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
The human body contains about 3,500mmol of potassium. Only about 2 per cent of this is in the extracellular fluid at any one time and the rest is intracellular. This 98 per cent is held in cells by a set of complex mechanisms and is pumped in and out of them by Na/K-ATPase pumps.
Potassium has many biological functions. It is a co-factor for many enzymes and it is required for insulin secretion, creatine phosphorylation, carbohydrate metabolism and protein synthesis. The ratio of intracellular to extracellular potassium is the major determinant of muscular and neuronal excitability and if this balance is disturbed, various pathological states can develop.
Tiny changes in the amount of potassium pumped in and out of cells can profoundly affect the level in the extracellular fluid and hence plasma levels. Such changes are often silent and insidious and, sometimes, it is not until a person collapses and is admitted to hospital that the problem is identified. Panel 1 lists factors causing the movement of potassium into cells.
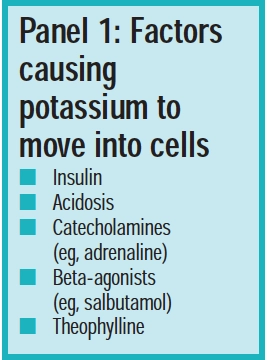
One factor affecting plasma potassium levels is blood pH. Acidosis causes potassium to move out of cells and alkalosis causes potassium to move into cells. Insulin is another important regulator. If the plasma potassium level rises, insulin release is stimulated and this, in turn, causes potassium to move into cells. This effect is due to insulin stimulating activity in Na/K-ATPase pumps.

Source: Andrew Lambert Photography/Science Photo Library
Potassium levels
With respect to potassium levels, there are two issues to consider: the amount in plasma and the total amount in the body. The reference range for plasma potassium is between 3.5 and 5.5mmol/L, but this can vary from one laboratory to another. Most ranges allow for two standard deviations from the mean. One in 20 patients will fall outside the range but this may be normal for them. Erroneously high plasma potassium levels can be measured if there is trauma when the blood is taken because cell damage allows intracellular potassium to be released into the sample. In secondary care, blood must not be taken from veins that have intravenous fluids running because this can also affect the reading.
The total amount of potassium in the body is controlled by the quantity ingested and eliminated and is not always reflected by plasma level, which also depends on the regulatory factors discussed above. For example, in patients with diabetic ketoacidosis high plasma potassium levels are seen although total body potassium may be low. When insulin is administered, plasma potassium falls rapidly and patients will need intravenous potassium supplementation. Indeed, these patients can suffer life-threatening hypokalaemia if their intravenous replacement fluids do not contain potassium.
Identify knowldge gaps
- Which drugs and diseases affect potassium levels?
- What role might potassium play in long-term conditions?
- Which foods are rich in potassium?
Before reading on, think about how this article may help you to do your job better. The Royal Pharmaceutical Society’s areas of competence for pharmacists are listed in “Plan and record”, (available at: www.rpsgb.org/education). This article relates to “common disease states” and “health education and promotion” (see appendix 4 of “Plan and record”).
Absorption and elimination
Potassium is derived from the diet and the reference nutrient intake is 90mmol per day. The form of the potassium does not affect its absorption and about 90 per cent of ingested potassium is absorbed, irrespective of the amount consumed. However, low dietary potassium intakes have been observed in the West and it is estimated that most women between the ages of 31 and 50 years in the US consume no more than half of the recommended daily amount of potassium. In the UK, the elderly and people of Afro-Caribbean descent have the least potassium in their diet.
Potassium is excreted in urine, faeces and sweat. The major route of elimination is via the kidneys. Potassium is freely filtered into the proximal tubule from the blood and as urine passes down the tubule it is almost completely reabsorbed. Once urine reaches the distal tubule potassium is passively secreted into the urine as a direct result of sodium reabsorption (ie, each sodium ion is swapped for a potassium ion to maintain neutrality, known as tubular secretion). Panel 2 gives a brief reminder of how the kidneys work.
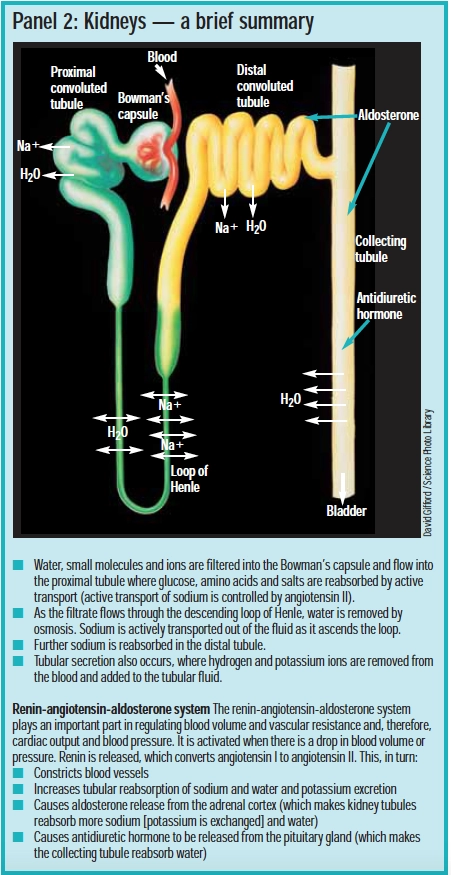
Neutrality can also be maintained by secretion of hydrogen ions. These are secreted preferentially in acidosis (in an attempt to increase pH) and potassium ions are preferentially secreted in alkalosis. Acidotic patients, therefore, tend to retain potassium and alkalotic patients tend to excrete potassium. However, excretion via the kidneys can be greatly affected by diuretics and drugs that affect the pH of the fluid in the kidney tubule itself (eg, amphotericin causes renal tubular acidosis, which increases potassium loss).
Excretion in sweat and faeces is usually negligible. Potassium is secreted into intestinal fluid. The colon contains about 30mmol/L but this is reabsorbed in the final part of the bowel, leaving faeces containing a small amount of potassium. Sweat contains about 5mmol/L potassium.
Hypokalaemia
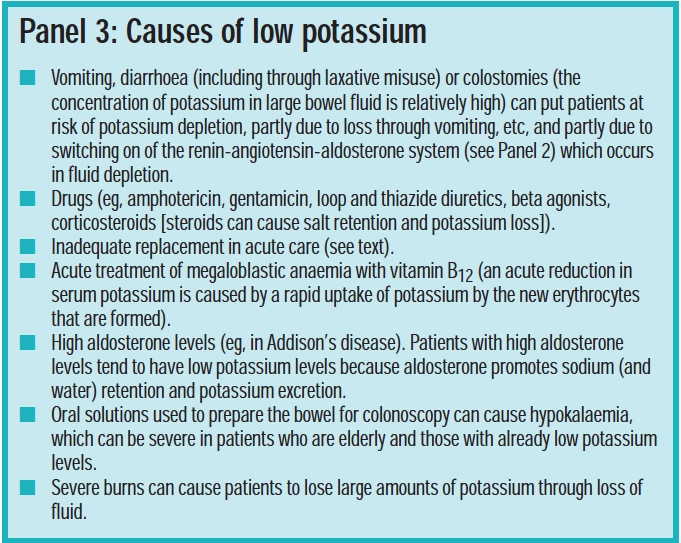
Although sweat contains little potassium, it is possible for hypokalaemia to occur through vigorous exercise in humid conditions. Panel 3 lists other factors that can lead to low potassium levels.
Hospital inpatients frequently experience low potassium levels due to inadequate supplementation or movement of potassium into the intracellular space rather than from renal losses.
Causes include endogenous catecholamine release (eg, after a heart attack) and medication. Some acute care drugs can cause enough potassium to move in to cells to cause symptoms of hypokalaemia but when the drug is stopped, potassium will move back out of the cells. Administration of intravenous glucose solutions also tends to reduce potassium plasma levels because potassium moves with glucose into cells, under the action of insulin.
In primary care, low potassium levels are likely to be caused by renal losses from activation of the renin-aldosterone-angiotensin system (see Panel 2) or by the use of drugs that increase potassium excretion. The most common drugs in use that increase potassium excretion are the thiazide diuretics (bendroflumethiazide, chlortalidone, cyclopenthiazide, indapamide, metolazone, xipimide) and loop diuretics (furosemide, bumetanide, torasemide).
In the past, combinations of diuretics were used to avoid hypokalaemia but these tended to cause low sodium levels and, for this reason plus cost issues, they are now prescribed less often. The fall in potassium in patients taking long-term thiazide diuretics is of the order of 0.3–0.5mmol/L. Patients taking a thiazide for hypertension should be prescribed the lowest dose (eg, 2.5mg of bendroflumethiazide or25mg of hydrochlorthiazide). With higher doses, low potassium levels are more likely and patients gain little more antihypertensive benefit.
Angiotensin-converting enzyme inhibitors (first-line to treat hypertension in patients under 55 years old) help to maintain potassium levels but, given the role of potassium in blood pressure (see below), it would seem sensible to recommend a high potassium intake for anyone taking a loop orthiazide diuretic alone.
Effects of low plasma potassium
Patients who have mild or moderate hypokalaemia (2.5–3.5mmol/L) are usually asymptomatic or only have only minor symptoms. Muscle weakness is the main symptom of low potassium, although arrhythmias, constipation and abdominal discomfort (paralyticileus), depression and confusion also occur.
Potassium is the primary ion mediating cardiac repolarisation and hypokalaemia is arrhythmogenic. All types of arrhythmia can occur, from atrial fibrillation to life-threatening ventricular tachycardia. Patients taking antiarrhythmic drugs are particularly at risk because hypokalaemia makes these drugs less effective. Digoxin, however, is an exception and its action (increased force of contraction and decreased conductivity) is potentiated if low plasma potassium levels are present, leading to increased signs of toxicity, such as vision disturbances and arrhythmias. Patients taking digoxin should, therefore, have their plasma potassium checked (we suggest annually) and corrected if low.
The possible long-term effects of low potassium levels are discussed in Panel 4.
Correcting hypokalaemia
Oral replacement is the method of choice for patientswith mild or moderate hypokalaemia. Indeed, this is the only option in primary care but it is not without its problems. Soluble tablets (eg, Sando K [12mmol/tablet]) or syrup (eg, Kay-Cee-L [1mmol/ml]) are the most effective choices. Patients need at least 60mmol per day for four or five days. However, some people find these products extremely unpalatable, due to a metallic aftertaste. Nausea, vomiting and abdominal pain have been reported after use and contribute to non-compliance. Flavourings may be added to the fluid but, in our experience, it is difficult to mask the aftertaste.
Another option is to use a slow release formulation, but these are less well absorbed and only contain 8mmol per tablet. In addition, slow release potassium products can cause intestinal ulceration — potassium is highly irritant and the tablets can lead to high local concentrations. They should be avoided in anyone with intestinal motility problems. The tablets should not be taken last thing at night and should be swallowed whole with plenty of fluid or food. For these reasons, slow release potassium tablets should only be used when liquid products cannot be tolerated. Advice is essential when they are supplied.
If cardiac arrhythmias or significant symptoms are present, more aggressive therapy is required. Intravenous potassium is frequently used in hospitals but it is extremely dangerous if mishandled.
Rates above 20mmol/h should only be used in critical care areas due to acute effects on the heart. Faster rates may be required in patients with severe hypokalaemia. Fast replacement may also be required before emergency surgery because low potassium levels make inhaled anaesthetics more likely to cause arrhythmias. In these cases, the prescriber must weigh up the risks. Hospital pharmacists need to ensure that products are available in appropriate strengths and that monitoring is undertaken.
In 2002, the National Patient Safety Agency required acute trusts to remove potassium ampoules from non-essential clinical areas due to the dangers from their misuse. Ready-made products (specials) are frequently bought by hospitals to prevent the need to use ampoules but these are also potentially dangerous — care must be taken over strength and infusions must be given slowly.
Many patients with low plasma potassium will also have low magnesium levels, which will also need correcting.
According to the British National Formulary, compensation for loss of potassium may be particularly necessary for:
- Those taking antiarrhythmic drugs
- Those with secondary hyperaldosteronism (eg, in renal artery stenosis)
- Those with excessive potassium loss in the faeces (eg, chronic diarrhoea)
- The elderly (but extra care is required with dosing because renal insufficiency is common in these patients)
- Those taking drugs that are known to causeloss of potassium (although this is not normally necessary with low doses of diuretics)
When potassium salts are used to prevent hypokalaemia, the usual dose is around 25 to 50mmol daily, in divided doses. In our experience, however, compensation is often not required. If undertaken, monitoring is necessary.
Hyperkalaemia
A potassium level that increases slowly is far better tolerated than one which suddenly increases. Patients with renal impairment need special consideration. They excrete less potassium in urine (more potassium is excreted in the faeces). Patients with renal disease can have plasma potassium levels over 6.0mmol/L with no signs or symptoms. Few effects may be noticed by the patient but slowed conduction within cardiac tissue and eventual conduction block can lead to asystole. Electrocardiogram changes usually occur well before asystole but ECG monitoring may not be possible in primary care. In addition to renal disease, causes of high potassium include:
- Recent tissue damage (eg, crush injuries)
- Heparin
- Low aldosterone levels
- Acidosis
- ACE inhibitors
- Aldosterone antagonists (spironolactone, eplerenone)
- Potassium-sparing diuretics (amiloride, triamterene)
Correcting hyperkalaemia
Mild hyperkalaemia is defined as a plasma potassium less than 6.5mmol/L, with no major symptoms, and is treated as described below. Severe hyperkalaemia (potassium above 6.5mmol/L, with major symptoms) should be treated urgently in hospital because there is a risk of arrhythmias. A plasma potassium level over 8mmol/L should be treated as an emergency. For all categories of hyperkalaemia, anyunderlying cause should be dealt with.
Mild hyperkalaemia
In mild hyperkalaemia, a polystyrene sulphonate resin, such as calcium resonium, should be given. The oral route is preferred (15g tds) because if the rectal route is used (30g in methylcellulose) the enema needs to be kept in the bowel for nine hours (the patient must not have a bowel movement during this time). It can also lead to rectal ulceration. Taken orally, the resin takes at least 24 hours to work and should be stopped as soon as the potassium level reaches 5.0mmol/L because the level will continue to fall until all the resin is cleared from the body. Laxatives may be needed because the resin causes constipation.
Severe hyperkalaemia
Treatment of severe hyperkalaemia falls into three phases and can only be carried out in hospital. First, cardiac stabilisation should be provided with 10ml (2.25mmol) calcium gluconate 10 per cent, given intravenously over five minutes. Second, a temporary reduction in serum potassium can be given by infusing 50ml of 50 per cent glucose with 15 units soluble insulin. The aim is to move potassium into cells. Plasma potassium will only continue to decrease for one to two hours after stopping the treatment. Thirdly, potassium should be removed from the body with a polystyrene sulphonate resin as described above. Haemofiltration or nebulised salbutamol can also be used.
Dietary advice
Normal dietary intake is more than sufficient to compensate for excretion — it is unusual for hypokalaemia to occur solely due to poor dietary intake. Similarly, increased intake rarely causes problems in the absence of other contributing factors. However, for people at risk of hypo- or hyperkalaemia, pharmacists can give dietary advice. In addition, the health benefits of a high potassium intake have been described in Panel 4. Panel 5 lists foods that are high in potassium.

A diet rich in fresh fruit and vegetables will contain good amounts whereas a diet high in processed foods is likely to below in potassium. Portion size, however, is important; a lot of something low in potassium can lead to relatively high potassium levels.
Food preparation can also affect potassium intake. The potassium content of food can be reduced by boiling in lots of water, a process known as “leaching” in renal diet handbooks.
The balance between sodium and potassium is important because excess sodium intake can deplete potassium. Another source of dietary potassium is the salt substitute potassium chloride. This is not inherently unsafe but use should be avoided in anyone taking medicines that can raise potassium or in patients with renal impairment — pharmacists should check medication histories before recommending it. Any dietary changes in chronic renal disease should be under the advice of a dietitian; potassium restriction is usually unnecessary in peritoneal dialysis but is often needed in patients on haemodialysis or before these therapies.
Action: practice points
Reading is only one way to undertake CPD and the Society will expect to see various approaches in a pharmacist’s CPD portfolio.
- Make sure patients with cardiovascular disease know about potassium rich foods and reinforce the message that low sodium and high potassium is good for the heart.
- When dispensing a potassium-lowering drug make sure the patient is aware of the benefit of a diet rich in potassium.
- Ensure you are familiar with hospital policy on the use of potassium ampoules and that appropriate potassium containing intravenous fluids are available.
Evaluate
For your work to be presented as CPD, you need to evaluate your reading and any other activities. Answer the following questions: What have you learnt? How has it added value to your practice? (Have you applied this learning or had any feedback?) What will you do now and how will this be achieved?
Further reading
- Halperin ML, Kamel KS. Potassium (electrolyte quintet series). The Lancet 1998;352:135–40, provides a good technical review of the role of potassium in body functioning.
- Sica DA, Struthers AD, Cushman WC, Wood M, Banas JS, Epstein MD. Importance of potassium in cardiovascular disease. Journal of Clinical Hypertension 2002;4:198–206.
- The Expert Group on Vitamins and Minerals has produced a risk assessment of potassium, available at: www.food.gov.uk (accessed 12 April 2007).
Correction
In Panel 1 (factors causing potassium to move into cells), the second listed factor should read alkalosis, not acidosis.


