DR P. MARAZZI/SCIENCE PHOTO LIBRARY
After reading this article, you should be able to:
- Describe the causes of gout and the role of the immune system;
- Know how to treat acute flares of gout;
- Know and be able to explain to patients why it is important to treat gout long term;
- Know about the ‘treat-to-target’ strategy;
- Advocate for the role of pharmacy in the education, treatment and management of gout patients.
Introduction
Gout is the most common form of inflammatory arthritis in the UK, with a prevalence of 2.49 out of every 100 people, and this is rising[1,2]. Gout is easily diagnosed and managed with cheap, readily available medicines but has traditionally not received as much attention as other forms of arthritis.
The National Institute for Health and Social Care (NICE) guidelines for the diagnosis and management of gout emphasise the need for healthcare professionals to engage with this condition and remain aware of the latest evidence and treatment options[3]. This article provides a summary of the causes and symptoms of gout, explains the treatment options, and considers opportunities for pharmacy teams to become more involved.
How does gout present?
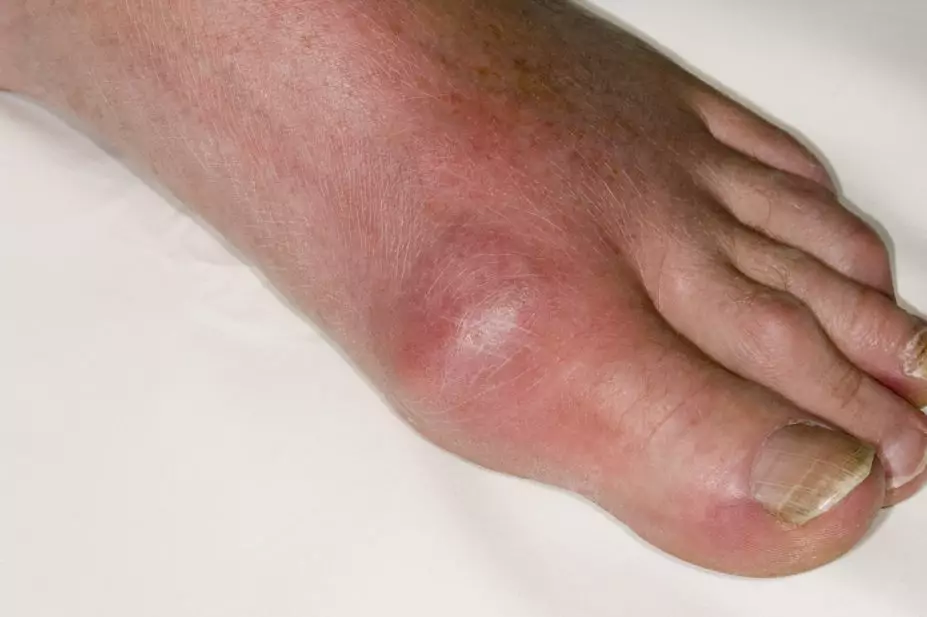
Gout typically presents as an acute (often overnight) onset of a severely painful red and swollen joint(s), but may also present with unexplained subcutaneous tophi (large bumps made of urate crystals) or chronic painful arthritis[3]. An initial acute gout attack classically affects one or both first metatarsal pharyngeal (MTP) joints, but subsequent acute flares can affect any joint in the body.
The joints most commonly affected by an acute gout attack are the first MTP joint, ankles, knees, elbows, wrists and fingers[4]. Between acute flares, gout causes the immune system to continue to react and there is an ongoing inflammatory process that, unless treated, continues indefinitely[5].
Causes of gout
Gout is a genetic disease, contrary to a widespread misunderstanding — both in the medical profession and general public — that it is a self-induced disease resulting from over-consumption[6,7]. Although there are factors that can exacerbate gout (clinical obesity, diet, alcohol and some medicines), they are not the underlying causes. The latest evidence base shows that gout is genetic, often familial, differs in prevalence between ethnicities and is caused by multiple combinations of genetic alleles[6].
Two genes have been identified as significant in causing gout: SLC2A9 and ABCG2(6). SLC2A9 affects excretion of urate from the kidneys and ABCG2 affects reabsorption rates of urate into the bloodstream and rate of release into the urine[6]. Both genes affect levels of urate in the blood and can lead to hyperuricaemia[6]. Other combinations of genes affect the response of the immune system to monosodium urate deposition in the joints, causing a T-cell response, which increases the risk of developing gout[5,8,9]. This immune response is then switched on in some of these at-risk individuals by some unknown environmental stimulus/insult, such as a virus, which triggers their innate immunity into attacking their own joints[9].
In summary:
- Gout is genetic;
- It only occurs in hyperuricaemic individuals who have a genetic predisposition to gout and whose innate immune response to urate crystals in their body has been triggered.
This knowledge tells us two important things:
- Hyperuricaemia is not a problem in all individuals, but only in those who develop gout;
- In individuals who develop gout their innate immune system is only active if they have hyperuricaemia.
These two crucial insights form the basis of how gout is treated[3,10,11].
Treatment rationale for gout
Often, a patient with gout will present with an acute flare and sudden, severe pain. Additionally, between acute flares many patients with gout will complain of arthritic pain and seek treatment; often such pain is ascribed to other conditions, such as osteoarthritis, and the patient is put on long-term painkillers rather than the long-term gout treatment that is required. The inflammatory effect of gout can also cause irreversible erosive damage to joints[12]. Patients with gout are also at higher risk of both morbidity and mortality, with increased incidences of diabetes mellitus, renal failure and other aspects of metabolic syndrome, including heart attack[13–18].
Diagnosis and management of gout
The first National Institute for Health and Care Excellence (NICE) guidelines on the diagnosis and management of gout were published in June 2022[3]. Previously, gout diagnosis and management in the UK relied on the British Society for Rheumatology’s gout guidelines, published in 2017[19].
The NICE guidelines advise a ‘treat-to-target’ (T2T) approach for gout, with the aim being to bring urate levels below clinically significant levels and, therapeutically, ‘cure’ the condition. Doing this essentially ‘switches off’ the immune system and stops the chronic, persistent inflammatory process of gout in the body[5,9,20].
The standard treatment is either allopurinol or feboxustat. It is important to educate the patient about gout and advise them that treatment is required for life. Acute flares should be treated with short courses of low-dose colchicine or oral non-steroidal anti-inflammatory drugs (NSAIDs)[3]. If using the latter, consider co-prescribing a proton pump inhibitor for gastric protection[3].
Using the T2T approach, the NICE guidelines advise a target serum urate of <360 micromole/L (6mg/dL). A lower target of <300 micromole/L (5mg/dL) is recommended in those who have tophi or chronic gouty arthritis, or in those who continue to suffer from frequent flares of their gout despite being at or below the 360 micromole/L target[3].
Patient education is vital and pharmacists should discuss the causes of gout with the patient in an evidence-based way. It is also recommended that patients with gout undergo an annual review of their treatment to ensure they remain at the T2T dose[3]. A review also provides the opportunity to reinforce the need for long-term treatment adherence. A review of treatment practices in NHS England showed that little or no follow up is offered to patients after a gout flare, resulting in patients diagnosed with gout but either not treated or not treated with a therapeutically effective T2T dose[3]. This situation is concerning, especially when this is an arthritis that can be therapeutically ‘cured’.
The evidence shows that using a T2T approach increases the number of acute flares at one year compared with usual care but reduces the number at two years[3]. Overall, this means that acute flares should be less frequent in the medium and long term for patients on an effective T2T dose, improving the patient’s morbidity and mortality[3,20]. The NICE guidelines suggest that treating gout using the T2T approach and providing patients with annual reviews is likely to be cost-effective[3].
How should we treat gout?
Gout is best considered as a chronic condition with acute-on-chronic flares. It is important to treat the underlying, persistent inflammatory response as well as the acute flares. Patient education is important, especially addressing the common misconception that gout is caused by patient actions and that lifestyle changes can cure or stop their gout. The NICE guidelines highlight that there is insufficient evidence that a specific diet can manage gout, but that, as with many conditions, excessive weight, clinical obesity and excessive alcohol consumption can increase the severity of the condition[10,19,20].
Long-term treatment with allopurinol or feboxustat should be offered to all patients[3]. This is typically recommended to be started two to four weeks after the acute flare but, in patients who are struggling with very frequent flares, the NICE guidance allows for long-term treatment to be started immediately[3]. In patients with major cardiovascular disease (such as previous myocardial infarction, stroke or unstable angina) feboxustat is contraindicated, so only allopurinol should be offered[3,21].
Using the T2T approach, allopurinol or feboxustat should be titrated until the serum urate target is reached[3]. For feboxustat, there are only two doses available and you should start with the lowest dose[3]. For allopurinol, the typical starting dose is 100mg once daily, and it is typically titrated in increments of 100mg; use lower doses in at-risk patients, such as those with significant chronic kidney disease[3]. In patients who do not reach target serum urate despite titration, or who are unable to tolerate the medication, try the second of the two long-term treatment options (provided there are no contraindications) before considering referral to specialist care.
When starting people on long-term treatment, discuss the risks and benefits of prophylactic medication to prevent acute flares. One study showed colchicine can be used to reduce the risk of flares, but with an increased risk of gastrointestinal upset compared with placebo[3,22]. There are no studies evaluating the use of NSAIDs or low-dose steroids to prevent flares as prophylactic medication. Because of this, NICE recommends that colchicine is offered as first-line prophylaxis against flares when starting or titrating patients on allopurinol or feboxustat[3]. This use of prophylaxis reduces the risk of flares and increases the chance patients will adhere to long-term gout treatment[3].
Irrespective of whether prophylactic medication is used or not, it is extremely important to advise the patient that they should continue with allopurinol or feboxustat if they develop an acute flare and that add-in treatment will be prescribed for their flare. Explaining that it is the gout that is causing the flare and not their medication is important, as these medicines are often inappropriately blamed[3].
Acute flares are best treated with either low-dose colchicine of 500 micrograms twice daily or with an NSAID, preferably co-prescribing an NSAID with a proton pump inhibitor for gastric protection[3]. If both colchicine and NSAIDs are contraindicated or not tolerated, a low-dose steroid is recommended[3]. There does not seem to be any difference in the ability of all three treatments to manage the main symptoms of an acute flare of joint pain, tenderness and swelling[3].
Role of the pharmacist
Patients often present to their pharmacist following an acute flare of gout. This is especially likely if they have had a previous flare. It is important to consider red flag symptoms, which could alert you to potentially dangerous conditions such as a septic joint, pseudogout or inflammatory arthritis, and exclude these[3,10,19]. If there is any suspicion of sepsis, the patient should be immediately referred for emergency care.
If pseudogout or inflammatory arthritis is suspected, the patient should be urgently referred to their GP. While treating the acute flare, it is important to check whether the patient is receiving long-term treatment for gout and, if not, explain to them why this is important and signpost them to their GP.
The NICE guidelines advise annual reviews for patients with gout to check that they are achieving their target serum urate[3]. Health economic modelling by NICE indicates that this is likely to be cost-effective in the medium to long-term for primary care[3]. Such a review could be incorporated into patients’ wider annual review for their long-term conditions. The review should also look at medicines that might predispose patients to gout flares and, as required, discuss with the patient switching to alternatives.
The pharmacy team is a central component of the primary care team, with many practices and primary care networks directly employing or sharing a pharmacy team. When treating gout, pharmacists can use their in-depth understanding of medicines and their interactions, helping to ensure a clear T2T pathway and shorter waiting times for patients. Pharmacists can also support patients in other ways: addressing acute flares; providing patient education; reiterating the importance of ongoing treatment adherence; long-term annual reviews; and initiating and titrating long-term medication. Gout treatment does not need to be doctor-centric and the pharmacy team can look for opportunities to encourage primary care to consider different ways of shared working that benefits patients.
Declarations of interest:
- AD has received monies for work with: Emotive, the National Institute for Health and Care Excellence gout guidelines advisory committee, Pfizer, Pharmacy Magazine, Royal College of GPs conferences, Royal Pharmaceutical Society, Spink Health, Tilray;
- AD is a trustee of UK Gout Society and ex-trustee of Primary Care Rheumatology and Musculoskeletal Medicine Society.
- 1Kuo C-F, Grainge MJ, Mallen C, et al. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2014;74:661–7. doi:10.1136/annrheumdis-2013-204463
- 2Smith E, Hoy D, Cross M, et al. The global burden of gout: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1470–6. doi:10.1136/annrheumdis-2013-204647
- 3Gout: diagnosis and management . National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ng219/resources/gout-diagnosis-and-management-pdf-66143783599045 (accessed Aug 2022).
- 4Gout. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/gout/symptoms-causes/syc-20372897 (accessed Aug 2022).
- 5Kienhorst LBE, van Lochem E, Kievit W, et al. Gout Is a Chronic Inflammatory Disease in Which High Levels of Interleukin-8 (CXCL8), Myeloid-Related Protein 8/Myeloid-Related Protein 14 Complex, and an Altered Proteome Are Associated With Diabetes Mellitus and Cardiovascular Disease. Arthritis & Rheumatology. 2015;67:3303–13. doi:10.1002/art.39318
- 6Butler F, Alghubayshi A, Roman Y. The Epidemiology and Genetics of Hyperuricemia and Gout across Major Racial Groups: A Literature Review and Population Genetics Secondary Database Analysis. JPM. 2021;11:231. doi:10.3390/jpm11030231
- 7Dumain T. 12 Myths About Gout That You Should Stop Believing Now. Creaky Joints. 2021.https://creakyjoints.org/about-arthritis/gout/gout-overview/gout-myths/ (accessed Aug 2022).
- 8Cronstein BN, Terkeltaub R. Arthritis Res Ther. 2006;8:S3. doi:10.1186/ar1908
- 9Cabău G, Crișan TO, Klück V, et al. Urate‐induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol Rev. 2019;294:92–105. doi:10.1111/imr.12833
- 10Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2016;76:29–42. doi:10.1136/annrheumdis-2016-209707
- 11FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020;72:744–60. doi:10.1002/acr.24180
- 12Wu M, Liu FJ, Chen J, et al. Prevalence and Factors Associated With Bone Erosion in Patients With Gout. Arthritis Care Res. 2019;71:1653–9. doi:10.1002/acr.23816
- 13Abbott RD, Brand FN, Kannel WB, et al. Gout and coronary heart disease: The framingham study. Journal of Clinical Epidemiology. 1988;41:237–42. doi:10.1016/0895-4356(88)90127-8
- 14Krishnan E, Baker JF, Furst DE, et al. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54:2688–96. doi:10.1002/art.22014
- 15Sheane BJ, Cunnane G. Tophaceous Gout and Chronic Kidney Disease. JCR: Journal of Clinical Rheumatology. 2007;13:293. doi:10.1097/rhu.0b013e3181571119
- 16Choi HK, Curhan G. Independent Impact of Gout on Mortality and Risk for Coronary Heart Disease. Circulation. 2007;116:894–900. doi:10.1161/circulationaha.107.703389
- 17Krishnan E. Long-term Cardiovascular Mortality Among Middle-aged Men With Gout. Arch Intern Med. 2008;168:1104. doi:10.1001/archinte.168.10.1104
- 18Kuo C-F, See L-C, Luo S-F, et al. Gout: an independent risk factor for all-cause and cardiovascular mortality. Rheumatology. 2009;49:141–6. doi:10.1093/rheumatology/kep364
- 19Hui M, Carr A, Cameron S, et al. The British Society for Rheumatology Guideline for the Management of Gout. Rheumatology. 2017;56:e1–20. doi:10.1093/rheumatology/kex156
- 20Perez-Ruiz F, Moreno-Lledó A, Urionagüena I, et al. Treat to target in gout. Rheumatology. 2017;57:i20–6. doi:10.1093/rheumatology/kex442
- 21Febuxostat (Adenuric): increased risk of cardiovascular death and all-cause mortality in clinical trial in patients with a history of major cardiovascular disease. Medicines and Healthcare products Regulatory Agency. 2019.https://www.gov.uk/drug-safety-update/febuxostat-adenuric-increased-risk-of-cardiovascular-death-and-all-cause-mortality-in-clinical-trial-in-patients-with-a-history-of-major-cardiovascular-disease (accessed Aug 2022).
- 22Borstad G, Bryant L, Abel M, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004;31:2429–32.https://www.ncbi.nlm.nih.gov/pubmed/15570646
3 comments
You must be logged in to post a comment.


I must take issue with some points made by the author of this article. Although there are no errors in what has been written, it is the omissions that concern me. Firstly, there is no mention that the primary target may not be the metatarsal joints. Secondly, there is an implication that the condition is almost always accompanied by painful swelling of joints and rubra. Any, or all of, these features may be absent. I have a patient who presented with painless swellings in the proximal metacarpal joints of several fingers in both hands. There was limited flexor and extensor movements, and there was no pain associated with these movements, and only two fingers could be fully extended. Blood tests ruled out osteo- and rheumatoid arthritis, and it was only when the patient was tested for blood oxalate that we could confirm a diagnosis of gout with tophi. The clinical example in the article is misleading by failing to mentioning painless presentation, not mentioning the metacarpal joints and that the deformities and that there may not be flare-ups, but still progressive.
Hello Henry. I am the senior editor for learning and research at the journal. Thanks very much for your comment. I have shared your feedback with the author of the article and will look at this closely with them. Thanks again.
Update: we have finished looking into your comment and are satisfied that the article does not need to be updated.