Shutterstock
A proof of concept study has shown how opportunistic testing in community pharmacies may help identify those with coeliac disease who would not otherwise have been tested.
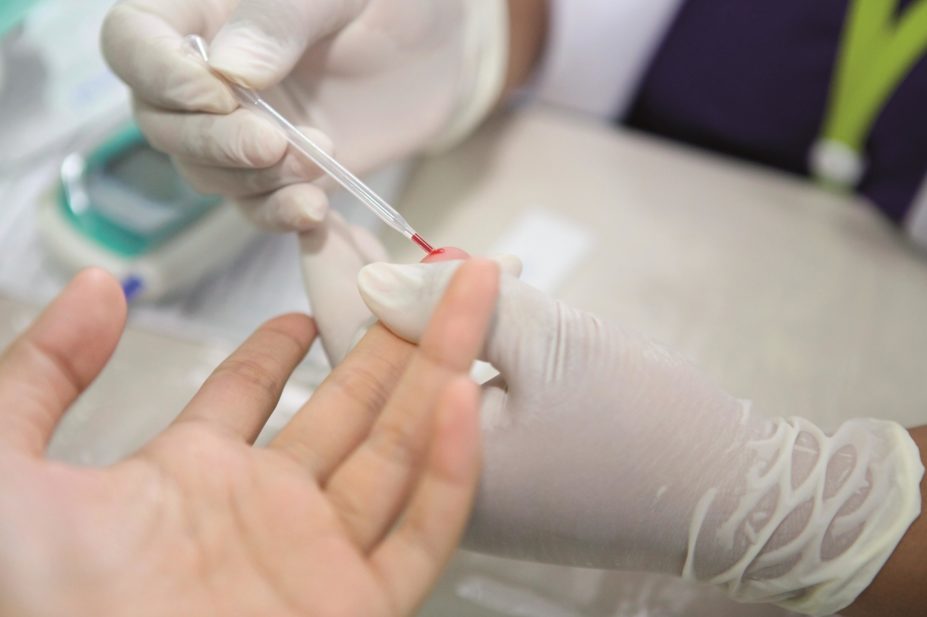
Community pharmacy customers requesting over-the-counter drugs or presenting prescriptions for medicines for the treatment of possible symptoms of coeliac disease, such as irritable bowel syndrome, diarrhoea and other general gastrointestinal problems and anaemias, were offered a free point-of-care finger prick test to check for total immunoglobulin A (IgA) and IgA tissue transglutaminase antibodies (IgA tTGA) — antibodies produced in coeliac disease.
Of the 551 people tested at 15 community pharmacies over six months, 52 (9.4%) tested positive for the antibodies, indicating that coeliac disease might be the cause of their symptoms. Those who tested positive were advised to make an appointment with their GP.
The researchers also found that 277 (50.3%) of those tested were accessing treatment for irritable bowel syndrome, while 142 (25.8%) were presented with diarrhoea.
Reporting their findings in the International Journal of Clinical Pharmacy
[1] (online, 8 August 2016), the researchers point out that while previous research has indicated that 1 in 100 people in the UK has coeliac disease, only 24% of those with the condition are diagnosed.
“This proof of concept study has shown that community pharmacies using a point-of-care test can effectively recognise and refer patients for confirmatory coeliac disease testing with high levels of customer and service provider satisfaction,” they say.
All 43 people who completed a satisfaction survey after being tested said that they would recommend the service to others, believing community pharmacy was a suitable place for testing. Participating community pharmacists also believed that the service enabled them to improve relationships with their customers and that local general practices were receptive to the service.
Comprehensive population screening is not recommended in the UK and targeted screening of individuals with related symptoms or associated conditions is recommended. This is predominantly carried out in general practice and involves serological tests for both IgA and IgA tTGA. Adults with a positive serological test result are then referred for endoscopy with biopsy to confirm or exclude coeliac disease.
James Kingsland, president of National Association of Primary Care, says: “Traditionally, the identification and implementation of targeted screening has solely been the role of primary care physicians. They are, however, only able to identify patients who present to them for treatment and advice.
“It is more usual for patients with general gastrointestinal symptoms to self-treat with therapy which is available from pharmacies and supermarkets, which is why community pharmacists are ideally placed to offer the finger prick testing, along with information and advice for those customers,” he says.
Sarah Sleet, chief executive of Coeliac UK, says she is “thrilled with the results of the study” but worries that plans to cut the community pharmacy budget and ongoing efficiencies within the NHS may impact on the roll out of the service.
However, she says: “The direct costs associated with undiagnosed coeliac disease are increased visits to the GP, use of medicines for symptomatic treatment, increased investigations and referral which all cost the NHS more in the long run, while the patient pays the price of reduced quality of life as misdiagnosis can mean an average wait of 13 years to get diagnosed.”
References
[1] Urwin H, Wright D, Twigg M et al. Early recognition of coeliac disease through community pharmacies: a proof of concept study. International Journal of Clinical Pharmacy 2016. doi: 10.1007/s11096-016-0368-4