Shutterstock.com
Two of the main medicines used for treating opioid addiction are equally safe and effective, according to a new, multi-centre study in The Lancet.
[1]
The federally funded, 24-week randomised controlled trial involved 570 patients addicted to opioids at eight US community-based inpatient facilities.
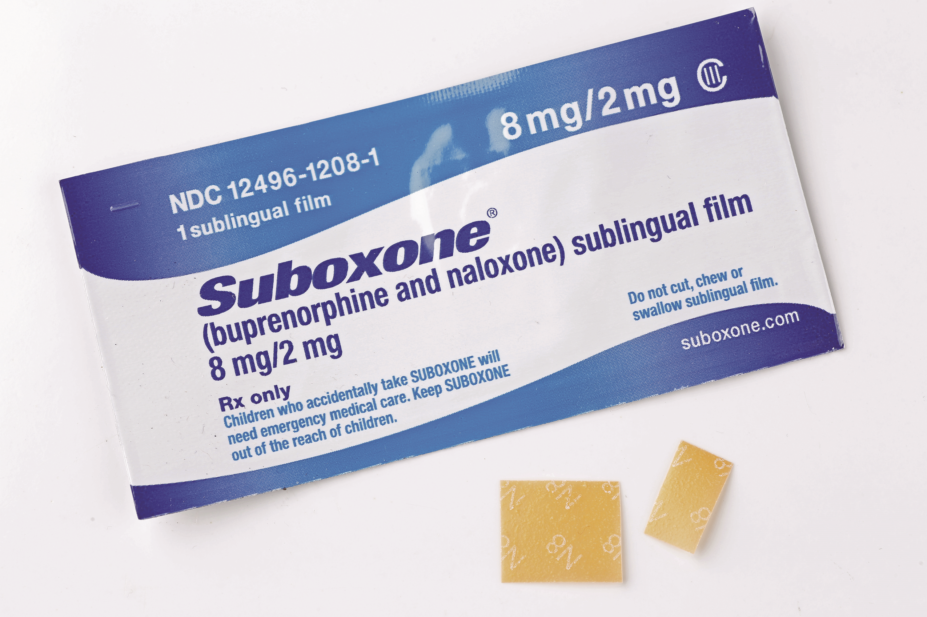
Subjects were randomly assigned one of two drugs used to prevent opioid relapse, extended-release naltrexone (XR-NTX), also known as Vivitrol, an opioid antagonist; or sublingual buprenorphine-naloxone (BUP-NX), also known as Suboxone, a partial opioid agonist.
The researchers found that 65% of patients who were given Vivitrol experienced relapses within the 24-week study period, compared with 57% of those on Suboxone.
Challenges with the drugs
Yet, the study found that Vivitrol posed a bigger challenge as the medication can only be given once a patient has been detoxed from opioids; consequently, more than a quarter of those in the Vivitrol group dropped out before taking their first dose of the medication.
For those in whom the treatments were successfully initiated, 52% of the Vivitrol group experienced opioid relapse, compared with 56% of the Suboxone group.
Taking into account only those participants successfully initiated on to the medication, the researchers noted: “24-week relapse events were similar across study groups”.
Side effects were similar across both groups, and self-reported opioid craving, while initially less in the Vivitrol group, was similar by the end of the study period. With the exception of mild-to-moderate injection site reactions for patients on Vivitrol, levels of side effects were similar among treatment groups.
The researchers concluded that further research should focus on improving the induction process for Vivitrol and on increasing retention rates for both medications.
Go-ahead for new device
Separately, the US Food and Drug Administration (FDA) has given marketing authorisation to the first device used to reduce the symptoms of opioid withdrawal.[2]
The NSS-2 Bridge, a small electrical nerve stimulator, is placed behind a patient’s ear. The device contains a battery-powered chip that emits electrical pulses to stimulate branches of certain cranial nerves, which is believed to provide relief from opioid withdrawal symptoms, such as sweating, gastrointestinal upset, insomnia and joint pain. Patients can use the device for up to five days during the acute physical withdrawal phase.
References
[1] Lee JD, Nunes EV et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X: BOT): a multicentre, open-label, randomised controlled trial. Lancet 2017. doi: 10.1016/S0140-6736(17)32812-X
[2] FDA grants marketing authorization of the first device for use in helping to reduce the symptoms of opioid withdrawal, FDA press release, 15 November 2017. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm585271.htm (accessed November 2017)
You may also be interested in

Voice of the Voiceless: co-produced materials to help reduce stigma for people receiving opioid substitution treatment in pharmacies

PJ view: The government’s approach to illegal street drugs should focus on treatment and prevention
