davide bonaldo / Alamy Stock Photo
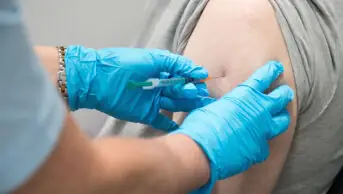
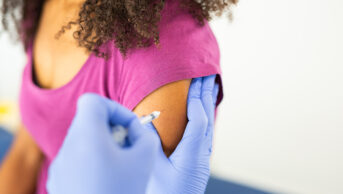
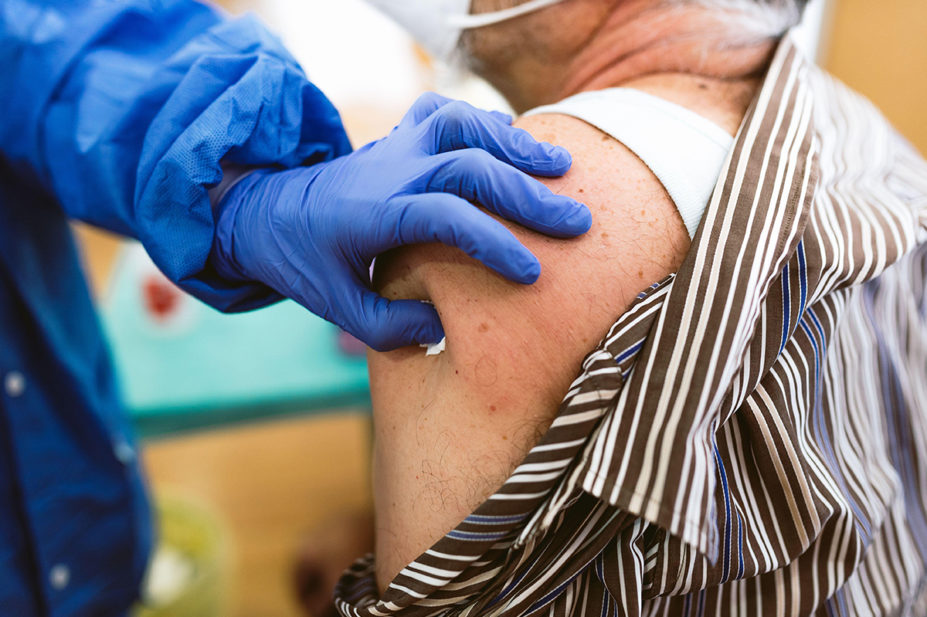
Patients aged over 50 years, as well as those at risk of serious disease, should be offered a booster dose of the COVID-19 vaccine from the week beginning 20 September 2021, government advisers have said.
The booster should be offered no earlier than six months after a patient’s second COVID-19 vaccination, with health secretary Sajid Javid confirming in a speech made in the House of Commons on 14 September 2021 that some patients would begin receiving offers for their additional dose from the following week.
In guidance issued on 14 September 2021, the Joint Committee on Vaccination and Immunisation (JCVI) said the additional dose would maintain a “high level of protection [against COVID-19] through the coming winter”.
The booster dose will be offered to all those who were vaccinated during ‘phase 1’ of the COVID-19 vaccination programme, in the same order of cohorts as was offered for the first two doses the of the vaccine.
This approach marks a change from interim guidance published by the JCVI in June 2021, which suggested the booster jab be offered in two stages.
“Persons vaccinated early during phase 1 will have completed their primary course approximately six months ago,” the guidance says. “Therefore, it would be appropriate for the booster vaccine programme to begin in September 2021, as soon as is operationally practicable.”
Addressing the House of Commons, Javid confirmed that booster jabs will be offered in line with the JCVI’s advice “from next week”.
“The NHS will contact people at the right time and nobody needs to come forward at this point,” he said.
The latest JCVI guidance also specifies a preference for the Pfizer/BioNTech vaccines to be administered as a booster jab, “irrespective of which product was used in the primary schedule”.
“Alternatively, individuals may be offered a half dose (50µg) of the Moderna vaccine, which should be well tolerated and is also likely to provide a strong booster response,” the guidance says.
Both the Pfizer/BioNTech and Moderna vaccines are mRNA-based COVID-19 vaccines.
“A half dose of Moderna vaccine is advised over a full dose due to the levels of reactogenicity seen following boosting with a full dose within the COV-BOOST trial,” the JCVI guidance explains.
The COV-BOOST trial, led by University Hospital Southampton NHS Foundation Trust and backed by government funding through the Vaccines Taskforce, has enrolled almost 3,000 participants to investigate which vaccines are most effective as a booster dose, depending on which vaccine was used to provide the initial two-dose course.
“Data from the COV-BOOST trial indicate that booster doses of COVID-19 vaccines are generally well tolerated and provide a substantial increase in vaccine-induced immune responses,” the guidance says.
“In particular, mRNA vaccines provide a strong booster effect, regardless of whether the primary course was with the Pfizer-BioNTech or the AstraZeneca vaccine.”
However, the JCVI advice adds that if either the Moderna or Pfizer/BioNTech vaccine “cannot be offered (e.g. due to contraindication)”, patients can be administered the Oxford/AstraZeneca vaccine.
Wei Shen Lim, chair of COVID-19 Immunisation for the JCVI, warned that the advice “does not imply that there will be a recurrent programme of booster doses every six months”.
“We will offer further advice regarding what to do when we come to perhaps a more steady state with regards to SARS COV 2 infections,” he said.
Lim added that the guidance “also does not imply that everybody who is otherwise healthy will necessarily need a booster dose”.
“We know from immunological principles that younger people tend to generate very good immune responses to primary course vaccination, more so than older people.”
Lim also urged eligible patients to receive a flu vaccine in addition to a COVID-19 booster jab.
“We have heard from the Medicines and Healthcare products Regulatory Agency and from clinical trial data that if it so happens that somebody is called up to have the COVID-19 vaccine and the flu vaccine on the same day, then it is safe to have both vaccines co-administered, usually in different arms … on the same day,” he said.
Commenting on the advice, Leyla Hannbeck, chief executive of the Association of Independent Multiple Pharmacies, said: “Community pharmacies are ready to play a full part in the delivery of COVID-19 booster jabs and must be called upon.
“With GPs already struggling to cope and busy [hospital emergency] departments, pharmacies should be used to the maximum this winter because we can help take the pressure off the NHS.”
Andrew Lane, chair of the National Pharmacy Association, said: “Pharmacies are well placed for delivering booster COVID-19 vaccinations, allowing GPs to focus on clearing the care backlog in the NHS.”
He added: “We need to examine the details of how the flu and COVID-19 vaccination programmes will run alongside each other, but pharmacies have proven themselves more than capable in relation to both.
“After the mass vaccination centres are wound down, pharmacies will still be there for the long haul out of the COVID-19 nightmare.”
Alastair Buxton, director of NHS services at the Pharmaceutical Services Negotiating Committee (PSNC), said it “is really pleased that a great many pharmacy contractors wanted to get involved in the COVID-19 booster programme and expressed their interest in this over the last few months”.
“NHS England and NHS Improvement have worked with those contractors to identify which are best placed to meet the forecast demand in local communities, alongside other vaccination sites.
“Given the amazing track record of the pharmacies already involved in the vaccination programme, we are sure the pharmacies joining the programme will deliver just what patients and the NHS need over the winter months to come.”
The JCVI advice has been issued separately to previous advice recommending a third COVID-19 jab for the severely immunosuppressed, with the JCVI expected to review whether this group requires a further booster at a later date.
Who will be offered a COVID-19 booster vaccine?
The booster vaccine will be offered to patients aged over 50 years as well as those at risk of serious illness, in the same order as they were offered the first two doses:
- Residents in a care home for older adults and their carers;
- People aged 80 years and over; frontline health and social care workers;
- People aged 75 years and over;
- People aged 70 years and over; clinically extremely vulnerable individuals
- People aged 65 years and over;
- People aged 16–64 years with underlying health conditions, which put them at higher risk of serious disease and mortality;
- People aged 60 years and over;
- People aged 55 years and over;
- People aged 50 years and over.
Source: Public Health England
READ MORE: COVID-19 booster campaign: everything we know so far