RICHARD USATINE MD / SCIENCE PHOTO LIBRARY
The UK Health Security Agency (UKHSA) has advised NHS clinical service providers to hold adequate stocks of appropriate personal protective equipment (PPE) in response to a heightened risk of clade 1 mpox virus (MPXV) outside of Central and East Africa.
The guidance, published on 15 August 2024, highlighted that all clinical services — including primary care, urgent care, sexual health services, paediatrics, obstetrics and A&E departments — should be aware of the public health information in the guidance.
This includes ensuring “isolation and management” of suspected cases in liaison with local infection prevention and control teams, and arranging discussions with local infection disease consultants for appropriate clinical management.
The guidance said that NHS providers should “ensure that they have adequate stocks of appropriate PPE and relevant staff are trained in its use for the assessment and treatment of patients presenting with suspected clade I MPXV infection”.
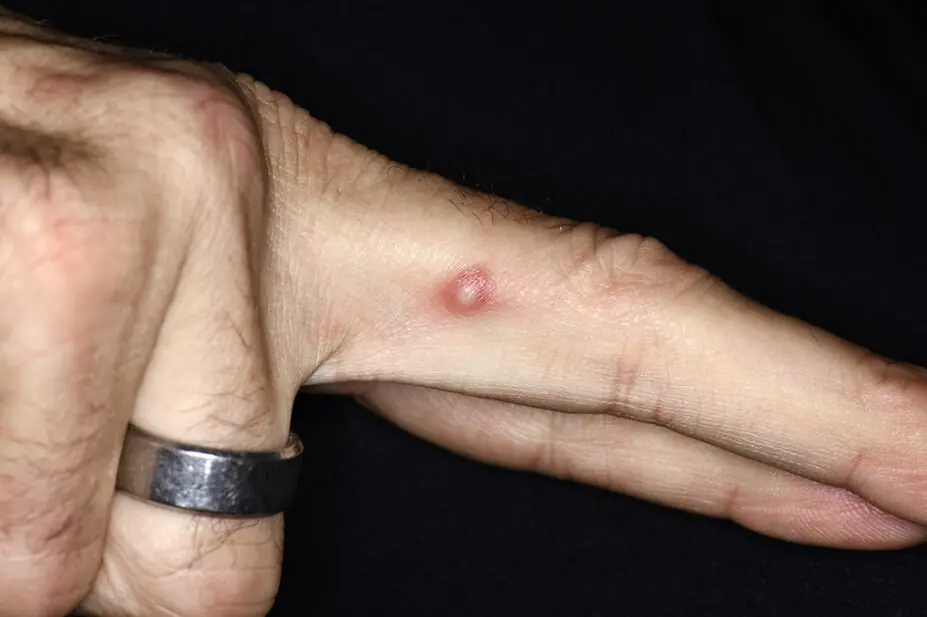
MPXV — formerly known as monkeypox — is a virus from the same family as smallpox that presents with a rash illness, which may be mild and localised, or severe and disseminated. There are two clades of the virus: clade II MPXV, which was responsible for the global outbreak that began in 2022; and clade I MPXV, which is considered more severe.
From 1 January 2022 to 30 June 2024, a total of 99,176 laboratory-confirmed cases of mpox have been reported to the UN health agency from 116 countries.
The UKHSA has instructed that all positive mpox samples must also be sent to its ‘Rare and Imported Pathogens Laboratory‘ for clade differentiating tests.
Symptoms of MPXV begin 5 to 21 days — on average 6 to 16 days — after exposure, with initial clinical presentations of fever, malaise, lymphadenopathy and headache. This is followed by a rash, often beginning on the face or genital area, which may spread to other parts of the body.
Anyone who has close contact with someone who has mpox is at risk of infection.
Treatment for MPXV is mainly supportive; however, owing to its severity, clade 1 MPXV is classified as a ‘high consequence infectious disease’ and requires management by infection specialists.
Commenting on the guidance Alastair Buxton, director of NHS services at Community Pharmacy England, said: “Whilst the likelihood of somebody presenting in a pharmacy with symptoms of mpox is currently low, all community pharmacy staff should be aware of and follow the guidance from UKHSA.
“The procedures are similar to those seen early in the COVID-19 pandemic so should be familiar to pharmacy teams,” he said.
On 14 August 2024, Tedros Adhanom Ghebreyesus, director general at the World Health Organization (WHO), declared that the emergence of the new clade I MPVX outbreak as a “public health emergency of international concern”.
Clade 1 cases have been reported in several countries in the African region — Democratic Republic of the Congo (DRC), Republic of Congo, Central African Republic, Burundi, Rwanda, Uganda, Kenya, Cameroon, Gabon.
Bordering countries at risk of exposure include Angola, South Sudan, Tanzania and Zambia.
According to WHO, 95% of cases reported in 2024 have been in DRC, with more than 15,000 clinically compatible cases and over 500 deaths reported at the time of going to press.
However, first case of clade 1 MPVX outside the African continent was confirmed by Sweden’s public health agency in August 2024.
In response to the WHO announcement, Meera Chand, deputy director at UKHSA, said: “The risk to the UK population is currently considered low. However, planning is underway to prepare for any cases that we might see in the UK.
“This includes ensuring that clinicians are aware and able to recognise cases promptly, that rapid testing is available, and that protocols are developed for the safe clinical care of people who have the infection and the prevention of onward transmission,” she added.
You may also be interested in
Norovirus and strategies for infection control

Case-based learning: insect bites and stings
