Stephen French / Alamy Stock Photo
The government’s advisory body on the control of dangerous drugs is looking into the safety of morphine oral solution, meeting minutes have revealed.
The minutes, covering a meeting held on 23 June 2021 and seen by The Pharmaceutical Journal following a Freedom of Information request, note that the Advisory Council on the Misuse of Drugs (ACMD) had recently received two regulation 28 notices “on pregabalin and short-acting liquid morphine solution” and were drafting responses.
Regulation 28 notices are sent by coroners after a person’s death to raise concerns with relevant bodies in an effort to prevent similar deaths in the future.
Following the notices, the minutes include an action for the ACMD’s technical committee, which provides advice on classification and scheduling of substances, “to seek and review information from the Department of Health and Social Care (DHSC) and NHS England and NHS Improvement (NHSE&I) on serious patient safety incidents involving morphine oral solutions”.
“Although this case might indicate a broader picture of abuse, it was important to be clear whether this was concerning poor clinical practice or criminal activity before seeing this as a problem.
“It was agreed that it would be appropriate for the technical committee to look into how common such incidents were and to seek further information from DHSC and NHSE&I,” the minutes added.
The Pharmaceutical Journal understands that the committee’s work on morphine oral solutions remains on its agenda.
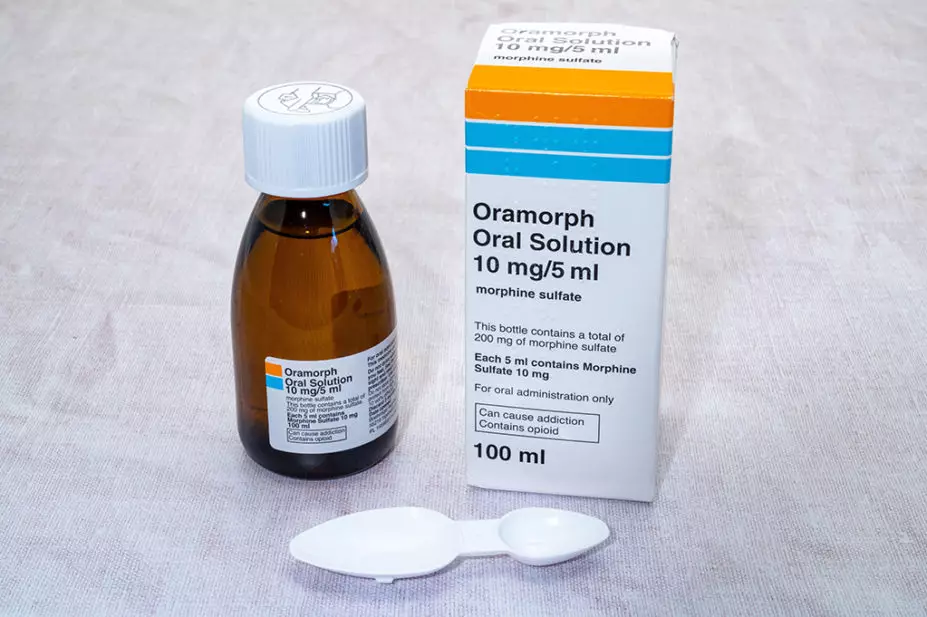
While the minutes do not specify which cases were being considered by the ACMD, an investigation by The Pharmaceutical Journal, published in September 2021, revealed that liquid morphine had contributed to the cause of death in at least three men and ten women in England and Wales since 2013, with coroners calling for tighter restrictions around its supply.
Liquid morphine is used to relieve severe pain, such as that associated with cancer, severe injury and surgery; however, under the Misuse of Drugs Act 1971, morphine sulfate 10mg/5mL oral solution is currently a schedule 5 controlled drug — as are codeine tablets and other diluted forms of schedule 2 drugs — unlike other forms of morphine, which are schedule 2.
The ACMD minutes also revealed a suggestion that it use regulation 28 notices as “a more systematic way to review trends in medical care”.
“Public Health England had been considering using regulation 28 notices as a source of data on abuse,” the minutes said.
A spokesperson for the Home Office told The Pharmaceutical Journal that it takes expert advice from the ACMD on classification of substances under the Misuse of Drugs Act 1971 and scheduling under the Misuse of Drugs Regulations 2001.
“While there are no plans at present to consider the rescheduling of oral morphine, we continue to work closely with the Department of Health and Social Care on understanding the complex factors involved in addiction and protecting individuals from these harms,” they added.
Read more: Investigation: should liquid morphine be reclassified?


