Shutterstock.com
A report on the government’s progress following the ‘Independent medicines and medical devices safety (IMMDS) review’ is awaiting publication, amid turbulence within the UK government, Henrietta Hughes, the first patient safety commissioner, has told The Pharmaceutical Journal.
The IMMDS Review report ‘First do no harm‘, published in July 2020, explored the impact of valproate, Primodos (norethisterone + ethinylestradiol; Schering) and pelvic mesh on women to whom they had been provided, and the children of women prescribed valproate.
The report, led by Baroness Julia Cumberlege, made several recommendations, including a call for a register of all women taking anti-epileptic drugs who become pregnant, as well as an independent redress agency for patients harmed by medicines.
Another recommendation was to establish an independent patient safety commissioner, a role appointed to Hughes, a GP and previously national guardian for the NHS, in July 2022.
According to the IMMDS review team, an update on the government’s progress with implementing the recommendations was expected to be published in summer 2022.
However, when asked during an interview with The Pharmaceutical Journal whether turbulence within the UK government is affecting work to improve patient safety, Hughes said: “There are reports, such as the ‘One year on’ report from ‘First do no harm’, which are awaiting review and publication.”
“Speaking to patients and patient advocates, they’re really looking forward to seeing the report published,” she added.
“The whole point of the [IMMDS] report was about incorporating the patient voice at every single stage, so if people are saying, ‘we’re waiting for the one-year report, we haven’t heard what’s happening with it, and we weren’t involved in the design and the delivery of the recommendations’ then I’m thinking to myself, ‘this is an even bigger piece of work’.”
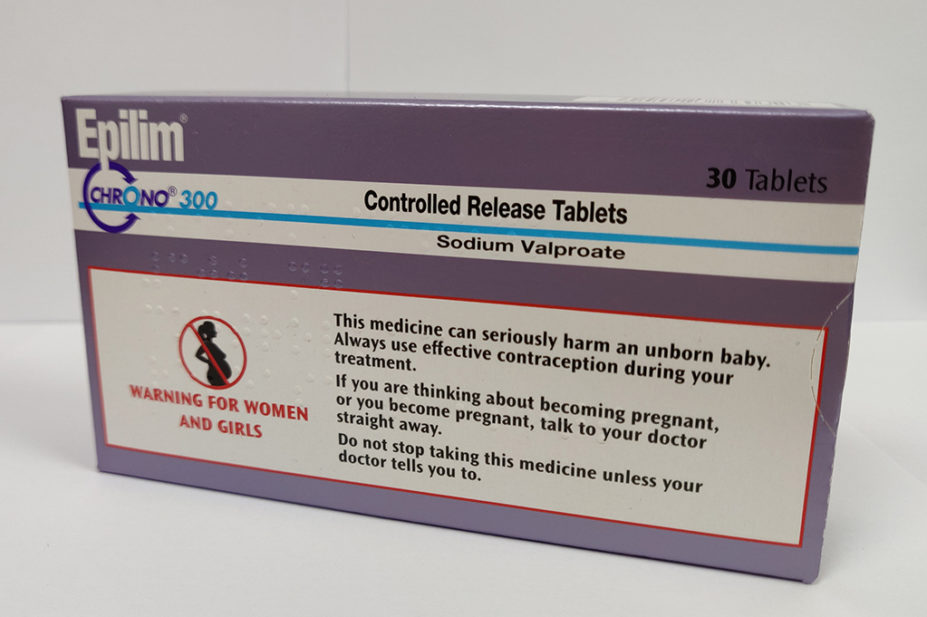
Hughes also said in the interview that, as patient safety commissioner, she wants “to go out and speak with pharmacists to find out what it is that’s stopping people getting the information that they need” on sodium valproate.
“In terms of the pregnancy prevention programme, one risk is that not everybody goes in person to pick up the medicine. With electronic prescribing and home delivery, we’re risking that one-to-one contact, between a person picking up their medication and their pharmacist.
“That is a really important relationship, and one that gives people the opportunity to ask questions and for safety concerns to be flagged,” she said.
An NHS England audit, published in August 2022, found that although 94.4% of patients were provided with advice and information about sodium valproate, more than 5% (675 people) were not.
Baroness Cumberlege, co-chair of the All-Party Parliamentary Group for ‘First do no harm’ and chair of the IMMDS Review, said she was “disappointed” to hear of the delay in the Department of Health and Social Care’s (DHSC’s) update on its implementation of her team’s recommendations.
“Day by day, those that have experienced avoidable harm linked to sodium valproate, Primodos and surgical mesh continue to suffer — and I call on the new government to commit to recognising their overwhelming need and to delivering justice for them, in the form of ex-gratia redress schemes.”
A spokesperson from the DHSC told The Pharmaceutical Journal the report would be published in due course and that it remained committed to implementing the accepted recommendations from the IMMDS Review.


