Shutterstock.com
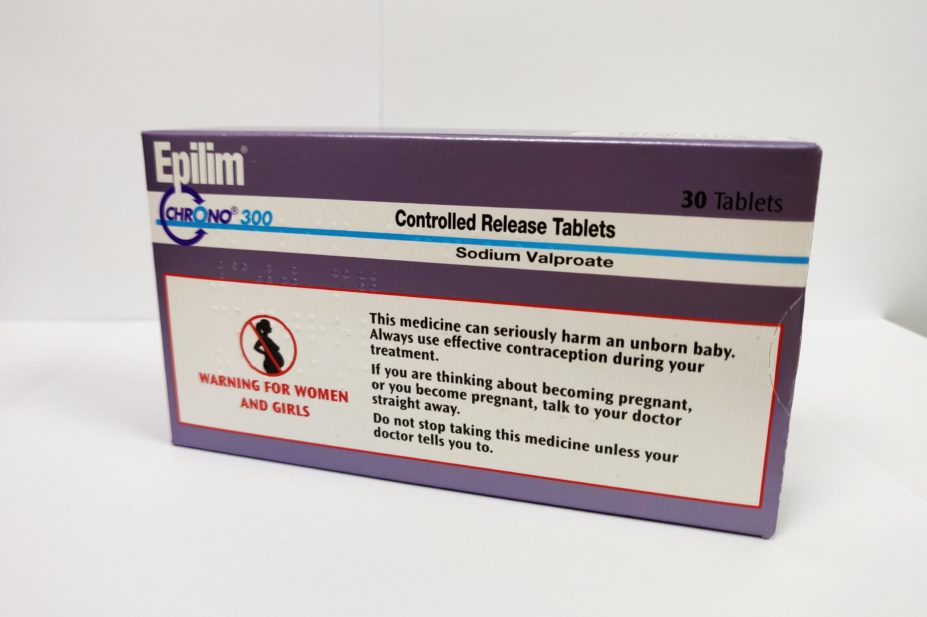
Just under half of pharmacists are not discussing the risks of taking sodium valproate when dispensing the drug to women, and just over half of pharmacists are not providing a patient card warning of the risks, a survey by three epilepsy charities has found.
Both these actions are recommended by the Medicines and Healthcare products Regulatory Agency (MHRA), which introduced the advice in 2018 owing to the risks of birth defects if the antiepileptic drug is taken during pregnancy.
The UK-wide survey of 514 women currently taking valproate, conducted by Epilepsy Action, the Epilepsy Society and Young Epilepsy, also found that just over one in ten (11%) women who take sodium valproate said they were unaware of the risks from taking it.
Simon Wigglesworth, deputy chief executive of Epilepsy Action, said it was “simply unacceptable that some women with epilepsy are still in the dark about the dangers of taking valproate in pregnancy.
“Just one woman unaware of the serious impact it could have on her unborn child is one woman too many. With a wealth of resources now available for health professionals to facilitate conversations, there is just no excuse for not explaining the risks to every woman taking valproate.”
The survey findings came the same day that the Independent Medicines and Medical Devices Safety Review published a report on the experiences of women who had been given valproate, Primodos (norethisterone/ethinylestradiol; Schering) or pelvic mesh.
The report found that “as data emerged on the risks of the use of sodium valproate over the decades, it took too long for action to be taken by the healthcare system”. It also said that “despite the efforts of the [pregnancy prevention programme], women are still becoming pregnant while [taking] valproate, without any knowledge of the risks”.
It also estimates that, each year, “hundreds” of babies are born “exposed to sodium valproate, despite the teratogenic risk being well recognised and undisputed”.
The report calls for a register of all women taking antiepileptic drugs who become pregnant, which would include mandatory data reporting on them and their children over lifetimes. It also says that all women currently on sodium valproate should be contacted for a medication review.
Baroness Julia Cumberlege, who chaired the review, said the report “makes for uncomfortable reading”.
“I have conducted many reviews and inquiries over the years, but I have never encountered anything like this; the intensity of suffering experienced by so many families, and the fact that they have endured it for decades,” she said.
“Much of this suffering was entirely avoidable, caused and compounded by failings in the health system itself.”
The report recommends the establishment of an independent patient safety commissioner and an independent redress agency for patients harmed by medicines. It adds that the MHRA needs “substantial revision” in terms of adverse event reporting.
Sandra Gidley, president of the Royal Pharmaceutical Society, said the review “makes important recommendations for improving the healthcare system’s ability to respond to concerns raised, and we will be reviewing these to see what we can do to contribute as the professional body for pharmacy.
“We know there continue to be problems associated with sodium valproate being prescribed for women and affecting unborn children. We are committed to working towards eliminating this problem and making the use of valproate safer as part of the Valproate Stakeholder Network.
“Pharmacists and their teams work hard to support patients every day, but we know there is more to do to make sure every woman who is prescribed valproate receives the valproate patient card every time it is dispensed and is counselled about the risks of taking it during pregnancy.”
In a statement, the MHRA said: “We recognise that patient safety must be continually protected and that many of the major changes recommended by the review cannot wait. We are therefore making changes without delay to ensure that we listen to patients and involve them in every aspect of our work.
“We are already taking steps to strengthen our collaboration with all bodies in the healthcare system and will strive to ensure that, working with these other bodies, the safety changes we advise are embedded without delay in clinical practice.”
For more information on valproate use in women and minimising the risks see this CPD resource, developed by The Pharmaceutical Journal alongside Sanofi.