Stephen Davies / Alamy Stock Photo
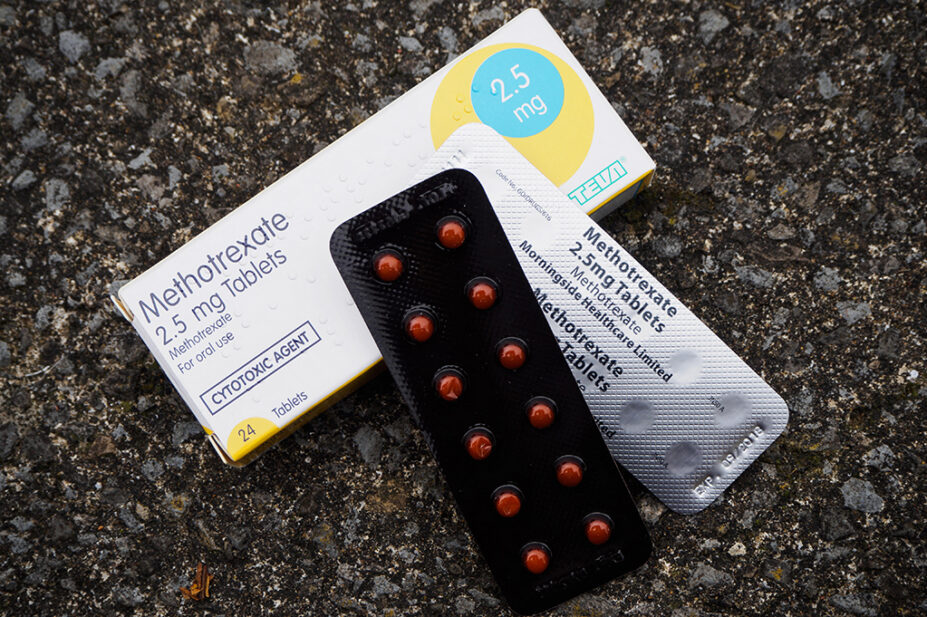
The pharmacy regulator has written to pharmacy teams after it received a concern that methotrexate had been dispensed with a label instructing a patient to take the medication once daily rather than once weekly.
Roz Gittins, chief pharmacy officer at the General Pharmaceutical Council, raised the issue in an email sent to pharmacists, pharmacy technicians and pharmacy owners on 18 December 2025 on emerging issues that had been raised with the regulator.
The email reminded pharmacy teams to supply the drug in line with a drug safety update from the Medicines and Healthcare products Regulatory Agency, published in September 2020.
The update said that teams should counsel patients and carers at the point of supply on once-weekly administration and the specific day of the week for dosing, while advising on risks of potential overdose.
Methotrexate, an immunosuppressant used in the treatment of cancers and autoimmune diseases, is most commonly prescribed for once-weekly administration owing to the risk of toxicity and serious adverse effects, such as organ and gastrointestinal damage.
The drug has been linked with serious and fatal overdoses owing to inadvertent once-daily administrations, with measures first brought in to minimise risks in 2006.
Gittins also raised a concern that a severely immunocompromised patient taking methotrexate received a supply of antibiotics through Pharmacy First.
Methotrexate taken in combination with some antibiotics can increase the risk of toxicity and myelosuppression.
She wrote that another contraindication issue had been reported, in which a patient who was routinely prescribed a long-term medication was provided with an antibiotic where an interaction was present.
“In some cases, such interactions can result in serious patient harm and, rarely, life-threatening outcomes,” Gittins said.
“While pharmacy teams may not always have access to a patient’s complete medication history, they should take all reasonable steps to ensure a safe and appropriate supply. This includes consultations, checking records where possible, providing counselling, and communicating with other healthcare professionals,” she added.
Gittins also raised the issue of propranolol toxicity.
“We are saddened to hear of further deaths and harm resulting from propranolol toxicity both through Prevention of Future Deaths reports and from our communications with stakeholders,” she wrote.
Gittins asked pharmacy teams to ensure they are aware of the risk of overdose from propranolol and consider further safety measures for at-risk patients, including highlighting the risks of overdose to patients and carers, discouraging stockpiling and identifying patients who may be vulnerable or at risk of self-harm and working with other healthcare professionals to ensure safe care.
She also wrote that the regulator had been made aware of fluoroquinolone antibiotic prescriptions despite them not being a first-line option, referring to a drug safety update published in January 2024, which restricted the use of systemic fluoroquinolones to situations when no other antibiotics are inappropriate, following reports of serious adverse reactions, such as tendonitis and tendon rupture.
Gittins reminded pharmacy teams to “remain vigilant” when encountering fluoroquinolone prescriptions and ensure their use is clinically justified.
She added that they should “provide clear counselling so that patients understand the potential risks and know to seek urgent medical advice if they experience symptoms suggestive of tendon, nerve, muscle or psychiatric adverse effects”.
Gittins urged pharmacy teams to review the email and ensure their IT systems support safe practice.
You may also be interested in

Patient safety commissioner to approach PM over ‘disappointing’ delay to valproate compensation

Medicines commission calls for greater clarity on risk of suicidal behaviour from antidepressants
