National Institute for Health and Care Excellence
For the first time, the National Institute for Health and Care Excellence (NICE) has asked a pharmaceutical company to provide evidence so that its anticancer drug can be considered for inclusion in the cancer drugs fund (CDF).
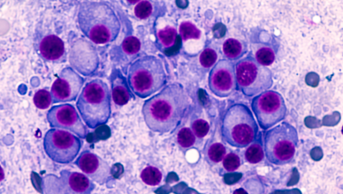
NICE, England’s health technology assessment body that provides guidance on which drugs should be used on the NHS, has approached Janssen to produce a case for adding ibrutinib (Imbruvica) to the CDF for the treatment of patients with chronic lymphocytic leukaemia with genetic changes – namely 17p deletion or TP53.
The unprecedented move comes just eight weeks after NICE was given control of the CDF under reforms announced by the government to make the fund sustainable.
In a statement, NICE’s independent advisory committee says it believes ibrutinib could benefit this specific group of patients who have not yet undergone any treatment.
Carole Longson, director of NICE’s centre for health technology evaluation, says: “As part of our commitment to give patients faster access to promising new cancer drugs, we have invited Janssen to submit a proposal to include ibrutinib in the CDF.
“If the company puts forward a proposal that is accepted by NICE and NHS England, conditional funding will be made available for ibrutinib in the CDF while more evidence is gathered to show how well it works. This will allow us to carefully monitor and evaluate the full benefits of ibrutinib and still ensure patients have access.”
Janssen said in a statement it is extremely disappointed with NICE’s decision not to recommend ibrutinib for use on the NHS.
“This recommendation means that once again, the UK lags well behind other European countries that have already opted to fund or reimburse the medicine for a patient population who have very few treatment options,” it added.