Shutterstock.com
The National Institute for Health and Care Excellence (NICE) has recommended a new drug for the treatment of moderately to severely active ulcerative colitis (UC).
Etrasimod (Velsipity; Pfizer), a selective sphingosine 1-phosphate (S1P) receptor modulator, is an oral pill, which patients take once daily. It reduces inflammation in the colon by helping to control the level of immune cells in the blood.
In a statement published alongside the final draft guidance, on 18 January 2024, NICE said it estimated “just over 25,000 people in England” would be eligible to receive the treatment, subject to licensing by the Medicines and Healthcare products Regulatory Agency.
The treatment will be offered to people with UC, who are aged over 16 years and have had an inadequate response, lost response or were intolerant to either conventional therapy or biological treatment.
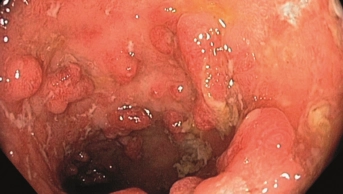
UC is a long-term disease, where the colon and rectum become inflamed, resulting in ulcers, bleeding and pus. It can cause recurring diarrhoea, arthritis and osteoporosis.
In its final draft guidance, NICE said etrasimod has not been directly compared in a clinical trial with usual treatments; however, indirect comparisons suggest that it is likely to work better than adalimumab (an immunotherapy treatment) and may be similarly effective to other usual treatments for moderately to severely active UC, which has not been previously treated with advanced treatment.
It added that, based on experience with other treatments used for this population with the same mechanism of action, it was considered likely that etrasimod would also be an effective treatment option.
In December 2023, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended the approval of a marketing authorisation for etrasimod in the European Union.
In October 2022, NICE recommended the use of ozanimod, an S1P receptor modulator, for the treatment of moderately to severely active UC.
Mohammed Allah-Ditta, an advanced gastroenterology pharmacist, specialising in inflammatory bowel disease medicines management at Calderdale and Huddersfield NHS Foundation Trust, said: “[Etrasimod] is a new drug in an existing class used in UC.
“The oral option, once-daily dosing and additional choice [of treatment option] are benefits for patients and clinicians.
“[However], in absence of head-to-head trials, [it’s] difficult to ascertain which drug is the right one for which patient,” he said.
Commenting on the announcement, Ruth Wakeman, director of services, advocacy and evidence at charity Crohn’s and Colitis UK said: “Around 300,000 people in the UK are living with UC, with over 12,000 new cases diagnosed every year.
“It is a lifelong condition, for which there is no known cure, and the symptoms are painful and debilitating.
“Expanding the treatment options for people living with colitis is a promising step forward, and we welcome NICE’s decision on etrasimod,” she added.
The final NICE guidance is scheduled to be published on 27 February 2024.