Chris Howes / Wild Places Photography / Alamy Stock Photo
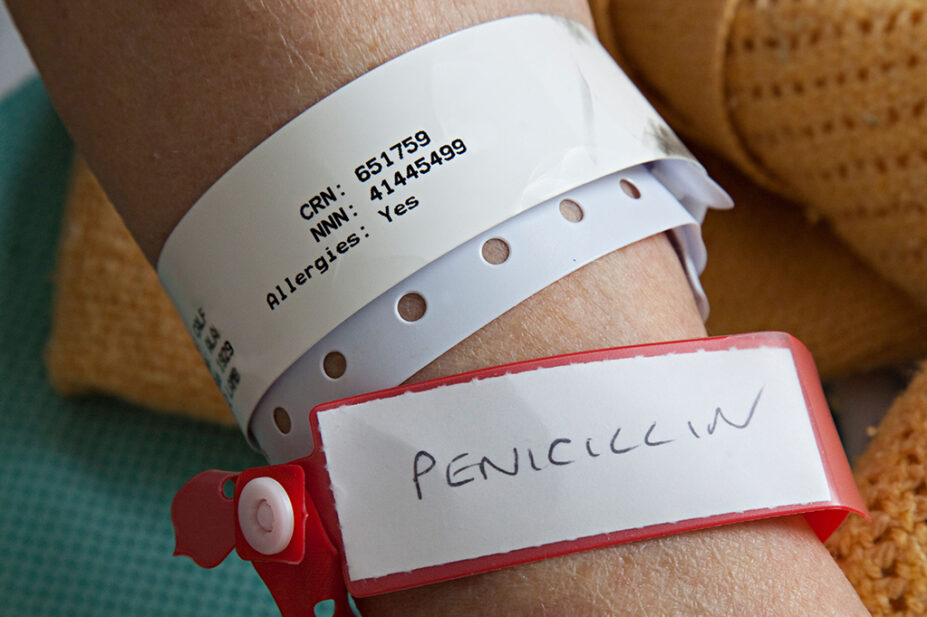
A Cornwall-based pharmacist is coordinating the UK’s participation in an international study examining the impact of removing incorrect penicillin allergy labels on patient outcomes.
Neil Powell, consultant antimicrobial pharmacist and associate director of antimicrobial stewardship at Royal Cornwall Hospital NHS Trust, is organising ten UK health services to submit data on the outcomes of antibiotic allergy de-labelling programmes to the ‘International Network of Antibiotic Allergy Nations’ (iNAAN) study.
The iNAAN study, led by Jason Trubiano, director of infectious diseases at the Centre for Antibiotic Allergy and Research in Melbourne, Australia, is examining the clinical effectiveness of multidisciplinary-led penicillin allergy assessment and de-labelling in hospitalised patients.
De-labelling — the removal of inappropriate patient allergy labels — could potentially improve patient outcomes, as people who are labelled as having a penicillin allergy are more likely to be treated with broad-spectrum antibiotics, which are linked to a higher incidence of antibiotic resistance and poorer health outcomes.
There are 30 hospitals across 8 countries — Australia, South Africa, Canada, Malaysia, Hong Kong, New Zealand, USA and the UK — taking part in the study, which began in 2022 and will run until 2026.
Royal Cornwall Hospital NHS Trust is planned to be the first UK health service to join the study in May 2024.
Powell told The Pharmaceutical Journal: “iNAAN is a big global data collection study, which is asking hospitals from around the world to submit data on all patients who get risk assessed for their penicillin allergy records.”
Patients with penicillin allergy records are included in the study when they are risk assessed, even if they are not de-labelled owing to being high risk.
High-risk patients include those who have had a severe reaction to penicillin in the past, such as facial swelling, fainting or a blistering skin reaction.
“Data will be collected on people with a penicillin allergy record, who have been de-labelled, and a cohort with a penicillin allergy record that hasn’t been de-labelled,” Powell explained.
“Then those patients can be case matched by age, gender and comorbidities, to see whether undoing the allergy label changes their health outcomes.”
Data will then be submitted to the iNAAN study via a point-of-care digital penicillin allergy toolkit embedded within a smart phone app, which has been designed specifically for participating health services.
Results of the iNAAN study are intended to inform national and international antibiotic allergy practice and antimicrobial stewardship policy.
Powell has also been conducting his own research into the best way to treat patients incorrectly labelled as allergic to penicillin since 2020, after successfully applying for a National Institute for Health and Care Research clinical doctoral research fellowship to develop and deliver his study.
The ‘Removing erroneous penicillin allergy labels’ (REPeAL) study is aimed at developing and implementing a structured set of questions and a decision support aid, to help clinicians decide whether to offer penicillin to those with an allergy record.
In September 2023, the Royal Pharmaceutical Society published a penicillin allergy checklist to help pharmacists and other healthcare professionals diagnose whether a patient is allergic to penicillin or not.