Shutterstock.com / Science Photo Library / MAG/The pharmaceutical Journal
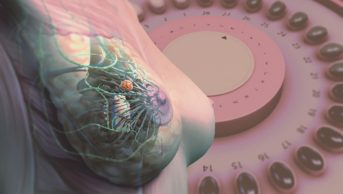
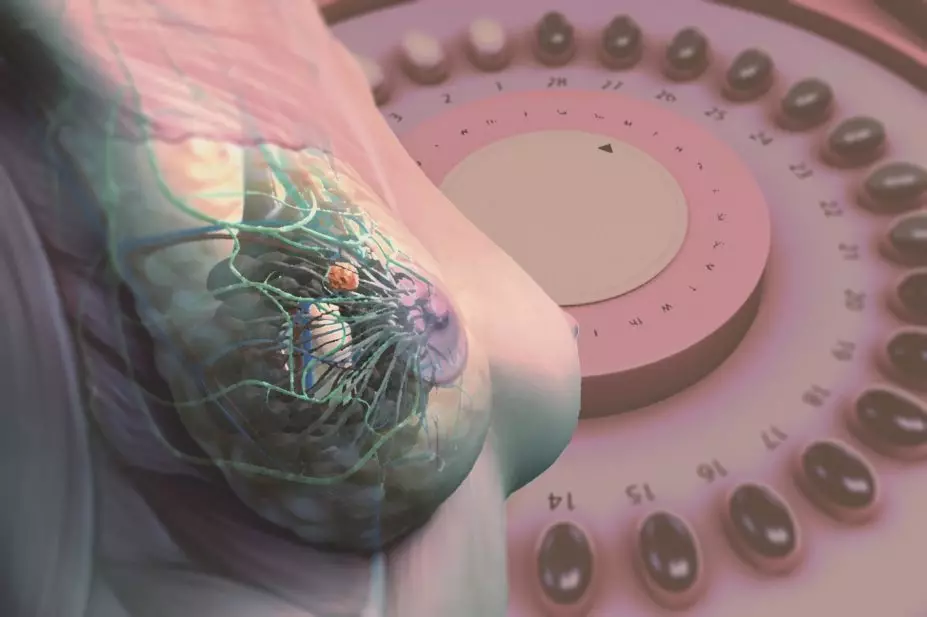
The purported benefits of hormone replacement therapy — now known as menopausal hormone therapy (MHT) in the medical community — are an improvement in general well-being during the menopausal period and protection against coronary heart disease, Alzheimer’s disease and osteoporosis. However, these claims are counterbalanced by documented concerns about an elevated risk for endometrial and breast cancer. Growth of both cancers is stimulated by oestrogen, but it is an increased risk of developing breast cancer that has hit the headlines, such as one article published in The Guardian in August 2016 — ‘British study finds risk of breast cancer nearly tripled by combined HRT’[1]
— which reported on a prospective study published in the British Journal of Cancer
[2]
.
Three months on, MHT is under the spotlight again for its safety in menopausal women. There is prima facie evidence that prescription practices for MHT reflect the incidence of breast cancer[3]
. Similarly, women taking MHT at the time of their breast cancer diagnosis experience a drop in breast tumour cell replication when MHT is stopped[4]
. Sex-steroid hormones seem to be “fuel for the fire” for the majority of breast cancers.
MHT in short courses has shown efficacy for the control of menopausal symptoms, whereas long courses of MHT have been examined in more detail as part of the Million Women’s Study (MWS) and the Institute for Cancer Research (ICR) study. The Women’s Health Initiative (WHI) showed that menopausal pathology did not improve, concluding that long courses of MHT should be avoided.
One exception for long-term MHT is in the treatment of osteoporosis, where MHT successfully builds bone. However, as a rule, it should only be used when other approaches have failed.
In this article, we will address the often conflicting statements concerning MHT and breast cancer incidence, many of which are reported inaccurately by the media. To achieve this, we will consider the three pivotal databases of MHT and briefly restate the primary findings with regard to breast cancer: the WHI[5]
,[6]
, the MWS[7]
and the recently published ICR report[8]
. These epidemiology studies do not answer the same questions but the conclusions of each study follow the same rules, dependent on knowledge of breast cancer biology. The complementary translational research uses models to decipher mechanisms to advance healthcare. The value of these pivotal studies[5],[6],[7],[8]
will be supported by a description of the successes and failures of the claims for the benefits of MHT[9]
. We will define biological rules known to control breast cancer risk, and emphasise the current recommendations for appropriate use of MHT.
The use of MHT to reduce negative symptoms of menopause is not new — it started clinically in 1942
Use of MHT in menopause
Menopause occurs when the ovary runs out of eggs. The slow collapse of reproductive ovarian function and steroidogenesis creates an immediate physiologic change in a woman’s quality of life, with frequent mood swings, hot flushes and night sweats. For contemporary women who have a longer life expectancy than previous generations, there are also some health concerns, including an increased risk of coronary heart disease and osteoporotic fractures, both of which have fatal consequences.
The use of MHT to reduce negative symptoms of menopause is not new — it started clinically in 1942[9]
. Initially, the primary source of cheap natural, orally active oestrogens was conjugated equine oestrogen (CEE). This would be used alone as MHT.
A trend towards use of oestrogen replacement at menopause skyrocketed in the 1960s to improve the quality of life for millions of women and it was even claimed that those taking it would remain youthful[10]
. However, during the preceding 20 years, the consequences of unopposed oestrogen actions and the benefits on women’s health were not documented in any appropriate clinical studies. Regulatory authorities did not require this at the time.
In 1975, an increase in endometrial cancer was documented in women using unopposed oestrogen therapy[11]
,[12]
. The solution was simple; prevent oestrogen-stimulated endometrial proliferation with the synthetic progestin medroxy-progesterone acetate (MPA). This was done cyclically to reduce endometrial cancer. In this way, the endometrium would be differentiated and withdrawal bleeding would occur to expel any cancerous tissue. As a result, MHT using CEE or oestradiol alone is used for hysterectomised women only, but treatment with oestrogen plus a synthetic progestin must be used for women whose uterus is still intact. This is a simple rule based on observational clinical experience. Nevertheless, not all target sites for oestrogen are regulated in the same way; a uterus is not a breast.
The best example of oestrogen-target site specificity in therapeutics is the discovery of selective oestrogen receptor modulators (SERMs)[13],[14]
. Tamoxifen — the pioneering SERM[15]
— provides the perfect example of the different target site specificity for the breast and the uterus. Tamoxifen stimulates pre-existing occult endometrial cancer and increases its incidence, but prevents the growth of breast cancer[16],[17]
.
Trial results
The ability of MPA to protect the uterus from oestrogen-induced cancer but enhance the risk of breast cancer at the same time is another case in biology. In the 20 years following the introduction of synthetic progestin to prevent endometrial cancer in MHT users, epidemiology identified an increase in breast cancer risk[18],[19],[20]
. At the same time, there was a growing belief that MHT would reduce the risk of coronary heart disease in women following menopause. This “belief” was again based on epidemiology. The WHI was conceived and executed to validate the hypothesis that MHT reduced coronary heart disease, and that the benefits of MHT outweighed the risks. This was important because more women die of coronary heart disease than of breast cancer. The results of the WHI did not support the benefits of MHT to reduce coronary heart disease[21]
or Alzheimer’s disease[22]
, but provided a unique insight into the biology of breast cancer.
There are rules for the development of breast cancer that are supported by results from clinical trials and epidemiology. The WHI[5],[6]
consisted of two placebo-controlled randomised clinical trials, which were conducted to find out whether CEE alone in hysterectomised women could reduce the risk of coronary heart disease and whether this was also true for women whose uterus was still intact who were given CEE and MPA. It is known that the risk of coronary heart disease increases with years past menopause. Therefore, women with an average age of over 60 years were recruited to enhance the statistical probability of seeing a benefit between MHT and placebo. Neither WHI trial showed a benefit in reducing occurrence of coronary heart disease[5],[6]
. In contrast, the MWS[7]
registered women in the “real world” who took MHT after menopause because of their need to reduce menopausal symptoms. The ICR study[8]
used serial questionnaires from the UK generations study, which ensure adherence is checked over time.
The WHI trial of CEE plus MPA was stopped because there was no overall benefit, specifically for coronary heart disease, but there was an increased risk of breast cancer[5]
. Subsequently, analysis of the WHI noted that women who took CEE plus MPA and continued for five or ten years had a high risk of breast cancer, with an estimated hazard ratio of 1.64 and 2.19, respectively[23]
.
In the MWS[7]
, women who started combined MHT at menopause had a relative risk (RR) of 2.04 for breast cancer but, if combinations of an oestrogen plus a synthetic progestin was started more than five years after menopause, the RR was 1.53. The ICR study, with its repeat questionnaire of continued MHT use, had an enhanced risk of breast cancer at just over five years of 2.74 (95% confidence interval [CI] 2.05–3.65). This further increased to 3.27 (95% CI 1.53–6.99) at more than 15 years of use[8]
.
However, to ensure that the synthetic progestin is not protecting the breast from oestrogen-stimulated tumour growth, but is protecting the uterus, one has to consider the breast cancer risk with oestrogen alone. Oestrogen is a known carcinogen for mammary cancer in animals, and the driver of breast cancer cell replication under laboratory conditions[24]
. This is why anti-oestrogenic therapies (tamoxifen and aromatases inhibitors) are so successful in saving lives following a diagnosis of breast cancer[25],[26]
.
Based on clinical experience with successful anti-oestrogenic treatments for breast cancer, the fact that the WHI study looking at CEE used alone demonstrated a sustained reduction in the incidence of breast cancer appeared to be a paradox that demanded an explanation[6],[27]
. However, no decrease in breast cancer was observed for these women starting CEE alone MHT at menopause[28]
. In contrast, Beral et al.
[29]
reported an increased RR of 1.43 if treatment with oestrogen was started at menopause, but no increase in breast cancer risk (RR 1.05) if oestrogen was started five years after menopause began. In the ICR study, there was no significant increase in breast cancer risk with oestrogen MHT with current use after 6.6 years. The rules for oestrogen administration and breast cancer risk are established based on the time of administrations after menopause: in the MWS and the ICR study, the majority of patients started at menopause and it had a small or no effect on breast cancer risk. The WHI recruited women with a mean age of 63 years (standard deviation±7) and the overall finding was a 30% decrease in breast cancer risk[27]
. These data are consistent with adjuvant anti-hormone therapy and laboratory studies.
Long-term oestrogen deprivation (LTED) clinically preselects new cell populations so the breast cancer can survive. However, LTED also exposes a vulnerability to physiologic oestrogen-induced apoptosis. This new biology of oestrogen action in breast cancer has been reviewed[30],[31],[32]
. Not only is there consistency of clinical reports to support the oestrogen-induced apoptosis mechanism for LTED breast cancer cell death, but the mechanism is well established in the referenced literature[33],[34],[35],[36],[37]
. However, the fact that synthetic progestins can reverse the benefits of oestrogen requires an explanation.
Pharmacology
The complex pharmacology of synthetic progestins is an important factor in understanding changes in breast cancer incidence. The synthetic progestin, MPA, used in the WHI, has weak glucocorticoid activity[38]
. Recently, glucocorticoids were shown[39]
to inhibit oestrogen-induced apoptosis and MPA was able to permit the survival of new breast cancer cell populations in combination with low concentrations of oestrogen. This would explain why MPA causes an increase in breast cancer incidence and reverses the active CEE alone[27],[39]
. However, the situation is different in the MWS and the ICR study[7],[8]
. The synthetic progestins were MPA and 19-nortestosterone derivatives: norethisterone and norgestrel. If one concedes that MPA can block oestrogen-induced apoptosis in patients who start MHT more than five years post menopause, then the use of a synthetic progestin that has no glucocorticoid activity could be of benefit. The synthetic progestins that are 19-nortestosterone derivatives do not have glucocorticoid activity, but have oestrogenic properties to stimulate breast cancer cell growth[40],[41]
. This inconvenient fact could increase breast cancer cell replication if MHT is given immediately after menopause. In contrast, the oestrogenic properties of 19-nortestosterone derivatives would be an advantage for women who are more than five years postmenopausal.
Only women with severe menopausal symptoms should be candidates for no more than two years of therapy
Benefits versus risks
The benefits of MHT lie solely in improved well-being and quality of life during menopause. There is no suitable substitute for oestrogen. Women without a uterus appear to be at less risk of increased breast cancer incidence with oestrogen alone. Only women with severe menopausal symptoms should be candidates for no more than two years of therapy. In contrast, for women with an intact uterus, the substitution of an elevation in breast cancer risk by having MHT with a combination of oestrogen plus a synthetic progestin to prevent endometrial cancer creates additional problems. Breast cancer is harder to detect than endometrial cancer, so a woman who avoids mammography adds to her risk of breast cancer and will have a late diagnosis. There is another problem in the detection of breast cancer; MHT increases breast density. The issue of the differing pharmacologies of the synthetic progestins also adds to the negative aspects of synthetic progestins use. Either a progestin with glucocorticoid activity (MPA) or oestrogen-like activity (19-nortestosterone derivatives) could enhance breast cancer promoting properties if administered at the wrong time. Unlike the databases from the WHI, the MWS and ICR rely on long-term MHT administration (five to ten years), which proves their point about increases in breast cancer risk. Current guidelines avoid this approach. The multiple dimensions of time from menopause, duration of MHT and progestin choice each create an increased risk of breast cancer that can be reduced by applying simple rules of care.
After looking at the evidence, the use of MHT can be summarised as using the lowest dose of MHT for the shortest time to ameliorate serious menopausal symptoms. Every three to six months, women should temporarily stop treatment to check whether it is necessary to continue MHT. Following these guidelines[42]
will result in the control of a woman’s quality of life, and have virtually no impact on breast cancer risk.
Key words: Menopausal symptoms, breast cancer, oestrogen, synthetic progestin, menopausal hormone therapy.
Balkees Abderrahman is a postdoctoral fellow at the Department of Breast Medical Oncology and V Craig Jordan is Dallas/Fort Worth living legend chair of cancer research, professor of both breast medical oncology and molecular and cellular oncology, both at the University of Texas MD Anderson Cancer Center in Houston Texas. Correspondence: vcjordan@mdanderson.org
Acknowledgments: This work was supported by the National Institutes of Health, MD Anderson’s Cancer Center support grant CA016672, Susan G Komen for the Cure Foundation under award number SAC100009, and Cancer Prevention Research Institute of Texas (CPRIT) for the STARs and STARs Plus Awards. V Craig Jordan thanks the benefactors of the Dallas/Fort Worth living legend chair of cancer research for their generous support.
References
[1] Siddique H. British study finds risk of breast cancer nearly tripled by combined HRT. The Guardian 23 August 2016. Available at: https://www.theguardian.com/society/2016/aug/23/combined-hrt-increases-breast-cancer-risk-nearly-300 (accessed October 2016)
[2] Jones ME, Schoemaker MJ, Wright L et al. Menopausal hormone therapy and breast cancer: what is the true size of the increased risk? British Journal of Cancer 2016;115:607–615. doi: 10.1038/bjc.2016.231
[3] Ravdin PM, Cronin KA, Howlader N et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007;356(16):1670–1674. doi: 10.1056/NEJMsr070105
[4] Prasad R, Boland GP, Cramer A et al. Short-term biologic response to withdrawal of hormone replacement therapy in patients with invasive breast carcinoma. Cancer 2003;98(12):2539–2546. doi: 10.1002/cncr.11836
[5] Rossouw JE, Anderson GL, Prentice RL et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288(3):321–333. doi: 10.1001/jama.288.3.321
[6] Anderson GL, Limacher M, Assaf AR et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701
[7] Beral V, Million Women Study C. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003;362(9382):419–427. doi: 10.1016/S0140-6736(03)14065-2
[8] Jones ME, Schoemaker MJ, Wright L et al. Menopausal hormone therapy and breast cancer: what is the true size of the increased risk? Br J Cancer 2016;115(5):607–615. doi: 10.1038/bjc.2016.231
[9] Rozenberg S, Vandromme J & Antoine C. Postmenopausal hormone therapy: risks and benefits. Nat Rev Endocrinol 2013;9(4):216–227. doi: 10.1038/nrendo.2013.17
[10] Perricone N. Forever Young Pocket Books; 2011.
[11] Ziel HK & Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med 1975;293(23):1167–1170. doi: 10.1056/NEJM197512042932303
[12] Smith DC, Prentice R, Thompson DJ et al. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med 1975;293(23):1164–1167. doi: 10.1056/NEJM197512042932302
[13] Jordan VC. Selective estrogen receptor modulation: a personal perspective. Cancer Research 2001;61(15):5683–5687. PMID: 11479197
[14] Jordan VC. Estrogen Action, SERMs and Women’s Health. London Imperial College Press; 2013.
[15] Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nature Reviews Drug Discovery 2003;2(3):205–213.doi: 10.1038/nrd1031
[16] Gottardis MM, Robinson SP, Satyaswaroop PG et al. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Research 1988;48(4):812–815. PMID: 3338079
[17] Fornander T, Rutqvist LE, Cedermark B et al. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet 1989;1(8630):117–120. PMID: 2563046
[18] Colditz GA, Hankinson SE, Hunter DJ et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med 1995;332(24):1589–1593. doi: 10.1056/NEJM199506153322401
[19] Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 1997;350(9084):1047–1059. PMID: 10213546
[20] Ross RK, Paganini-Hill A, Wan PC et al. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst 2000;92(4):328–332. PMID: 10675382
[21] Manson JE, Hsia J, Johnson KC et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349(6):523–534. doi: 10.1056/NEJMoa030808
[22] Craig MC, Maki PM & Murphy DG. The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 2005;4(3):190–194. doi: 10.1016/S1474-4422(05)01016-1
[23] Prentice RL, Chlebowski RT, Stefanick ML et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol 2008;167(10):1207–1216. doi: 10.1093/aje/kwn044
[24] Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res 2009;69(4):1243–1254. doi: 10.1158/0008-5472.CAN-09-0029
[25] Early Breast Cancer Trialists’ Collaborative Group: Davies C, Godwin J, Gray R et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8
[26] Early Breast Cancer Trialists’ Collaborative Group: Dowsett M, Forbes JF, Bradley R et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1
[27] Anderson GL, Chlebowski RT, Aragaki AK et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol 2012;13(5):476–486. doi: 10.1016/S1470-2045(12)70075-X
[28] Prentice RL, Chlebowski RT, Stefanick ML et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol 2008;167(12):1407–1415. doi: 10.1093/aje/kwn090
[29] The Million Women Study Collaborators: Beral V, Reeves G, Bull D et al. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst 2011;103(4):296–305. doi: 10.1093/jnci/djq527
[30] Jordan VC. Linking estrogen-induced apoptosis with decreases in mortality following long-term adjuvant tamoxifen therapy. J Natl Cancer Inst 2014;106(11). doi: 10.1093/jnci/dju296
[31] Jordan VC. The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer. Endocr Relat Cancer 2015;22(1):R1–31. doi: 10.1530/ERC-14-0448
[32] Abderrahman B & Jordan VC. The modulation of estrogen-induced apoptosis as an interpretation of the womens health initiative trials. Expert Rev Endocrinol Metab 2016;11(1):81–86. doi: 10.1586/17446651.2016.1128324
[33] Yao K, Lee ES, Bentrem DJ et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res 2000;6(5):2028–2036. PMID: 10815929
[34] Song RX, Mor G, Naftolin F et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst 2001;93(22):1714–1723. doi: 10.1093/jnci/93.22.1714
[35] Lewis JS, Meeke K, Osipo C et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst 2005;97(23):1746–1759. doi: 10.1093/jnci/dji400
[36] Ariazi EA, Cunliffe HE, Lewis-Wambi JS et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci USA 2011;108(47):18879–18886. doi: 10.1073/pnas.1115188108
[37] Fan P, Cunliffe HE, Maximov PY et al. Integration of downstream signals of Insulin-like growth factor-1 receptor by endoplasmic reticulum stress for estrogen-induced growth or apoptosis in breast cancer cells. Mol Cancer Res 2015;13(10):1367–1376. doi: 10.1158/1541-7786.MCR-14-0494
[38] Stanczyk FZ, Hapgood JP, Winer S et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev 2013;34(2):171–208. doi: 10.1210/er.2012-1008
[39] Sweeney EE, Fan P & Jordan VC. Molecular modulation of estrogen-induced apoptosis by synthetic progestins in hormone replacement therapy: an insight into the women’s health initiative study. Cancer Research 2014;74(23):7060-–7068. doi: 10.1158/0008-5472.CAN-14-1784
[40] Jeng MH, Parker CJ & Jordan VC. Estrogenic potential of progestins in oral contraceptives to stimulate human breast cancer cell proliferation. Cancer Research 1992;52(23):6539–6546. PMID: 1423300
[41] Catherino WH, Jeng MH & Jordan VC. Norgestrel and gestodene stimulate breast cancer cell growth through an oestrogen receptor mediated mechanism. Br J Cancer 1993;67(5):945–952. PMID: 8494728
[42] Food and Drug Adminstration. Menopause and Hormones: Common Questions (updated 2015). Available at: http://www.fda.gov/ForConsumers/ByAudience/ForWomen/ucm118624.htm (accessed 20 October 2016)