Introduction: Post-intensive care syndrome (PICS) is associated with an increased risk of mortality. Critical illness recovery clinics have been recommended by the Faculty of Intensive Care Medicine (FICM) as an approach to manage the complexity of PICS. The COVID-19 pandemic has provided many healthcare challenges, not least how to support those who have survived critical illness related to the virus. Recent guidance from the FICM also recommends pharmacist involvement in rehabilitation and recovery clinics for patients discharged from hospital following COVID-19 critical illness. This evaluation was undertaken to determine the impact of the inclusion of a clinical pharmacist in this setting.
Method: All patients discharged following a COVID-19-related intensive care unit (ICU) admission in the Belfast Health and Social Care Trust, Northern Ireland, from 26 March 2020 to 15 May 2020 were offered enrolment in a rehabilitation and recovery programme. This involved an initial 6-week remote review, followed by a multidisciplinary face-to-face assessment at 12 weeks post-discharge. Each healthcare professional at the clinic contributed to an overall treatment plan, which was communicated to the patient’s GP. The clinical pharmacist carried out a structured medicines optimisation review and identified medication-related interventions, which were recorded and graded according to the Eadon criteria. Cost savings resulting from these interventions were estimated using the model described by the University of Sheffield School of Health and Related Research (ScHARR), a type of economic modelling that uses literature-based values of the costs of medication errors and compares this with the benefits of different medication-related interventions.
Results: A total of 42 patients were discharged during this period following COVID-19 ICU admission, with 93% (n=39) agreeing to enrolment in the follow-up clinic. Medication-related interventions were identified in 82% (n=32) of the 39 patients. The most common medication-related intervention was patient education, accounting for 38% (n=24) of all interventions.
Discussion: Analgesics were the class of medications most associated with requiring an intervention and 65% of interventions were graded as significant, resulting in improved care standards (i.e. Eadon ≥grade 4). Clinical pharmacist interventions yielded potential savings of £4.20–£8.59 per £1 invested, based on total potential savings in the range of £6,204–£12,699, with a total pharmacist investment of £1,478.
Conclusion: Clinical pharmacists have a role in medicines optimisation for patients recovering from COVID-19-related critical illness. Most interventions by a clinical pharmacist in a post-ICU recovery clinic were related to symptom management and patient education. Their role in this setting can help rationalise medicines and improve patients’ understanding, resulting in potential healthcare-related cost-savings and safer patient-centred care.
Keywords: Clinical pharmacy, COVID-19, critical care, integrated care, intensive care, interventions, medicines optimisation, outpatient clinic.
Original submitted: 20 November 2020; Revised submitted: 26 February 2021; Accepted for publication: 3 March 2021.
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus
Key points
- Post-intensive care syndrome describes the combination of persistent physical, cognitive and psychological impairments following prolonged ventilation;
- Emerging evidence suggests people who have survived COVID-19 critical illness require additional support with managing longer-term complications;
- Clinical pharmacists, as part of a multidisciplinary outpatient recovery clinic, can contribute to optimising medicines post-discharge;
- Most medicine-related interventions within this setting were classified as significant and were related to symptom management and patient education;
- Clinical pharmacist interventions yielded potential savings of £4.20–£8.59 per £1 invested, based on total potential savings in the range of £6,204–£12,699, with a total pharmacist investment of £1,478;
- Further studies are required to validate these results and evaluate long-term patient outcomes.
Jump to:
Introduction
Millions of patients are admitted to intensive care units (ICUs) each year, one third of whom require mechanical ventilation[1]. Advances in critical care mean an increasing number will survive; however, many develop health problems related to their illness, ventilator or other treatments. The rise in survivorship has created a growing population suffering from long-term consequences of critical care.
Post-intensive care syndrome (PICS) describes the combination of persistent physical, cognitive and psychological impairments present in patients at 12 months following prolonged ventilation[2]. While reports of PICS are common, its prevalence is hard to define because of differences in study patient populations, patient comorbidities and measurement tools, and the duration varies from one month to eight years[3].
In 2016, a multi-centre prospective cohort study with 406 patients identified that PICS was present in 64% and 56% of survivors at 3 and 12 months, respectively[4]. The most common new impairment at 3 and 12 months was cognitive impairment (38% and 33%, respectively). Depression was present in around one third of survivors at 3 and 12 months, whereas disability was present in 26% of survivors at 3 months and in 21% at 12 months. Common clinical manifestations include fatigue, anxiety, sexual dysfunction and sleep disturbances[3].
Following ICU admission, patients with PICS have an increased mortality rate and many do not achieve full recovery[5]. Less than 10% of patients on mechanical ventilation for more than four days are alive and fully independent one year later[3].
Many factors have been shown to improve critical care recovery, including preventive strategies, such as the ABCDE bundle. This is an evidence-based, inter-professional, multi-component approach that aims to reduce the risk for ICU-acquired delirium and weakness by reducing and/or removing sedation and mechanical ventilation therapy in a standardised process, while simultaneously promoting delirium monitoring and early mobility[6].
Daily sedation breaks in ICU, combined with physical and occupational therapy in the earliest days of critical illness, has shown better functional outcomes and resulted in a shorter duration of delirium[7].
Post-ICU psychological programmes have also shown promise in improving patients’ and caregivers’ ability to cope by providing support, counselling and education on stress management. The involvement of a psychologist in post-discharge care has been shown to reduce the prevalence of depression, post-traumatic stress disorder and anxiety by half[8–10].
In the UK, the National Institute for Health and Care Excellence (NICE) published a clinical guideline focused on ‘Rehabilitation after critical illness’ in 2009[11]. The guideline recommends that patients receive a review of rehabilitation, health and social needs two to three months following discharge from critical care. This review should comprise a functional assessment of physical and non-physical sequelae of critical illness and provision of support where needed[11].
Although the guideline was successful in highlighting the importance of rehabilitation for survivors of critical illness, there has been limited development of this clinical service across the UK since its publication. In 2013, a survey of adult ICU units in the UK (n=182) found only 27% (n=48) offered a follow-up service 2–3 months following hospital discharge[12]. Lack of funding was reported as the most frequent (n=149/164, 90%) and main barrier (n=99/156, 63.5%) to providing services[12].
Quality standards associated with critical care rehabilitation were subsequently published by NICE in 2017[13]. They recommend healthcare professionals (such as nurses, intensive care professionals, specialists in rehabilitation medicine, physiotherapists and clinical psychologists working in critical care follow-up clinics) carry out a review two to three months after discharge from critical care for adults who were in critical care for more than four days and at risk of morbidity. Outcomes should include identification of physical/non-physical problems and levels of satisfaction with support received to manage rehabilitation needs among adults discharged from critical care[13].
One model that can be used to identify people with PICS and coordinate the necessary services is post-ICU clinics[14]. Teams within this type of clinic can liaise with GPs, who may have limited resources to manage the patient’s complex needs post-discharge home, which itself may range from a relatively simple hospital ward discharge to a complex prolonged period in intermediate care, followed by an extensive home care package.
The goals of the clinic model are to prospectively identify impairments and create individualised restorative plans. Although quantitative studies are weak, qualitative reports suggest a clear benefit, with the clinic model considered to be the important determinant in terms of patient outcome. Inter-disciplinary teams, including pharmacy input, are associated with longitudinal improvements in outcomes at 2.5-month intervals[15]. Critical illness recovery outpatient clinics offer the interpersonal quality of professional consultation in addition to the opportunity for clinical examination and measurement of same-day investigations, if required[7].
The patient’s return to the post-ICU outpatient clinic can also be an excellent opportunity to review the appropriateness of current medication. A cohort study of consecutive patients seen in an ICU follow-up clinic in Scotland found that medication-related problems following an ICU admission were common, occurring in 81% (n=38) of the patients studied. The most common documented problem was drug omission 29% (n=20). Just under two-thirds of pharmacist interventions at the clinic were likely to improve therapeutic benefit or avoid serious side effects[16]. Studies within post-ICU clinics in the United States have also demonstrated the positive contribution of clinical pharmacists, with interventions focused on reducing deliriogenic agents and improving compliance with patient education[17,18].
A novel strain of coronavirus SARS-CoV-2 was first detected in December 2019 in Wuhan, a city in China’s Hubei province[19]. On 11 March 2020, the World Health Organization officially declared the outbreak a pandemic and COVID-19 has rapidly become an international public health emergency, with a significant impact on both patients and healthcare systems[20].
Patients diagnosed with COVID-19 have a broad range of presentations, from asymptomatic carriers to those with severe critical illness with pneumonia, acute respiratory distress syndrome and multi-organ failure[21]. Risk factors for increased disease severity include obesity, cancer, type 2 diabetes, chronic kidney disease and COPD[22–24].
Those who have survived COVID-19-related critical illness during a pandemic surge may have distinct challenges for recovery including:
- Impact of lockdown/social distancing on usual support networks and social scaffold;
- Emotional stressors associated with sudden isolation, personal protective equipment, heightened attention/coverage and incomplete knowledge of illness;
- Prolonged waiting times to access specialist healthcare services with reduced capacity during the pandemic[25].
While clinicians are becoming familiar with acute management and some specific complications of COVID-19 illness, the longer-term sequelae of this disease remain unknown. Emerging evidence from the initial stages of the pandemic suggest that a large proportion of COVID-19 patients discharged from hospital were still experiencing symptoms of breathlessness, fatigue, anxiety and depression two to three months after contracting the virus[26–28]. Extended cohort studies are needed to better understand long-term disease consequences.
UK-based initiatives — such as the Post-hospitalisation COVID-19 study (PHOSP-COVID) and Research and Innovation for post COVID-19 Rehabilitation (RICOVR) — are currently investigating these longer-term effects[29,30]. While PICS is well recognised and there is a body of evidence describing medication-related problems in ICU survivors to date, little is known about these problems in the context of COVID-related critical illness[31].
In May 2020, the Faculty of Intensive Care Medicine (FICM) produced guidance on the recovery and rehabilitation for patients following the pandemic[25]. The guidance recommends that pharmacists should be part of the multidisciplinary team (MDT) delivering the assessments and interventions required in such a clinic.
Aim and objectives
The aim of this study was to evaluate the role of a clinical pharmacist in a COVID intensive care recovery clinic.
The objectives were to:
- Provide a pharmacist-led medication review for each patient attending the clinic;
- Identify and classify medication-related interventions, which could be graded according to significance;
- Assign cost savings associated with medication-related interventions based on potential reduction of healthcare resource utilisation.
Methods
The Belfast Health and Social Care Trust is one of five health trusts in Northern Ireland. It provides local services to the 340,000 people in Belfast, while also providing specialist services for the entire population of Northern Ireland. During the initial surge of hospital admissions relating to the first wave of COVID-19 infection (26 March 2020 to 15 May 2020), 46 patients in the trust required critical care treatment.
The trust established a pilot COVID ICU recovery service to provide comprehensive, multidisciplinary follow-up for all patients who had required critical care admission related to COVID-19 infection. The service was initially set up on 27 April 2020 in preparation for the first cohort of COVID-19 patients to be discharged from critical care. Given the complexity of COVID-19-related PICS, the overall aim was to enhance safe and effective post-discharge patient care and to improve recovery. The pilot focused on supporting GPs by providing an integrated follow-up service coordinating medical care and rehabilitation.
Of the original 46 patients treated in Belfast Trust COVID ICU, 42 patients were discharged during the initial pandemic surge (March–May 2020) and were invited to enrol in the rehabilitation and recovery programme, with three patients (7%) opting out. A hybrid model of virtual and in-person clinics was established with the initial six-week consultation carried out remotely by an ICU consultant, nurse and clinical psychologist. The programme continued with a collaborative, interdisciplinary rehabilitation clinic at 12 weeks post-discharge. This secondary care clinic, which took place in a hospital outpatient setting, was led by an intensive care consultant and with input from an ICU nurse, a speech and language therapist, a physiotherapist, a dietitian, a medical secretary, a clinical pharmacist (band 8a) and a clinical psychologist.
FICM identified five main outpatient service archetypes that have evolved across the UK in recent years for critical illness recovery services:
- 1:1 uniprofessional appointment;
- Panel MDT;
- Carousel MDT;
- Group or cohort;
- Drop-in café[25].
In addition, service models are strongly influenced by the needs of the local case mix and available resources, as well as by the professional background of the founding practitioner(s). For the Belfast Trust, the hybrid model, as described previously, was chosen to allow patients a remote review during the early stages of recovery, followed by a face-to-face MDT clinic with a carousel model (i.e. each MDT member in a different room). This 12-week clinic provided the opportunity for clinical examination, measurement and same-day investigations (see supplementary file 1).
If they wish, the patient can also revisit the critical care unit. This allows them to meet the staff who cared for them, improve understanding of what happened and help them process the critical care experience[32].
At this follow-up clinic, each healthcare professional had a face-to-face consultation with the patient lasting around 30 minutes. At the end of each clinic session, team members would meet to discuss action plans for each patient. The collective assessment and recommendations formed from these consultations allowed the team to develop a comprehensive treatment plan to ensure optimal transition and appropriate follow-up of outstanding issues. This was communicated to the patient’s GP in the form of a clinic summary letter sent via the electronic care record (see supplementary file 2)[33].
The evaluation below focuses on the clinical pharmacy intervention set within this follow-up clinic 12 weeks after hospital discharge.
Role of the clinical pharmacist
Pharmacy input for the clinic was provided by an Agenda for Change band 8a clinical pharmacist currently working in critical care with several years’ experience across a range of specialities.
- Attendance at follow-up clinic (09:00–17:00 with 30 minutes lunch break) every Tuesday for six weeks = 45 hours;
- Preparation time of 3 hours per clinic for the 6 clinics = 18 hours;
- Total = 63 hours; this equates to £1,478*.
*(Based on 2- to 3-year pay point HSC 2020–2021 band 8a salary of £45,753/year)[34].
During the 12-week post-discharge clinic:
- Medicines reconciliation was undertaken, including pre-admission, post-ICU discharge and post-hospital discharge;
- Patient interviews were conducted as part of a structured medicines optimisation review. This included medication history, adherence and discussion around concerns about their medicines. Identification of any medication-related interventions;
- Clinical interventions were recorded and graded according to the Eadon criteria, a scale ranging from 1–6, where grades ≥4 indicate a significant intervention resulting in improved standards of patient care, which ultimately prevent major organ damage to the patient or even death[35];
- Patients were provided with an individualised written guide to medicines, if required.
The primary outcome of this evaluation was to quantify the number of pharmacist interventions per visit. Secondary outcomes included categorising these interventions and classifying significance using the Eadon criteria. The grading of each intervention was independently checked for consistency by a consultant pharmacist within the trust who had experience using this criterion. This check resulted in two of the interventions changing grades, from criteria 3 to 4. On review by the pharmacist in the clinic, the grading was amended as per the suggestion. Cost savings resulting from interventions that prevent medication errors and adverse drug events have been estimated using the model described by the University of Sheffield School of Health and Related Research (ScHARR)[36].
Results
A total of 42 patients were discharged from Belfast Trust hospitals between 26 March 2020 and 15 May 2020 following COVID-19 ICU admission. Of these patients, 39 agreed to enrol in the follow-up service and all were reviewed by the clinical pharmacist at the 12-week clinic. The median length of stay in ICU was 12 days, with an interquartile range (IQR) of 8.5–22 days. Seventy-seven per cent were male, with a median age of 56 (IQR range 51–63 years). During their ICU admission, 100% (n=39) of patients required mechanical ventilation; 21% (n=8) required renal replacement therapy; and 66% (n=26) received cardiovascular support.
The clinical pharmacist provided a total of 64 interventions, with 46% (n=18) of patients requiring two or more interventions and 18% (n=7) requiring three of more interventions. Table 1 shows the number of medicines prescribed at different stages.
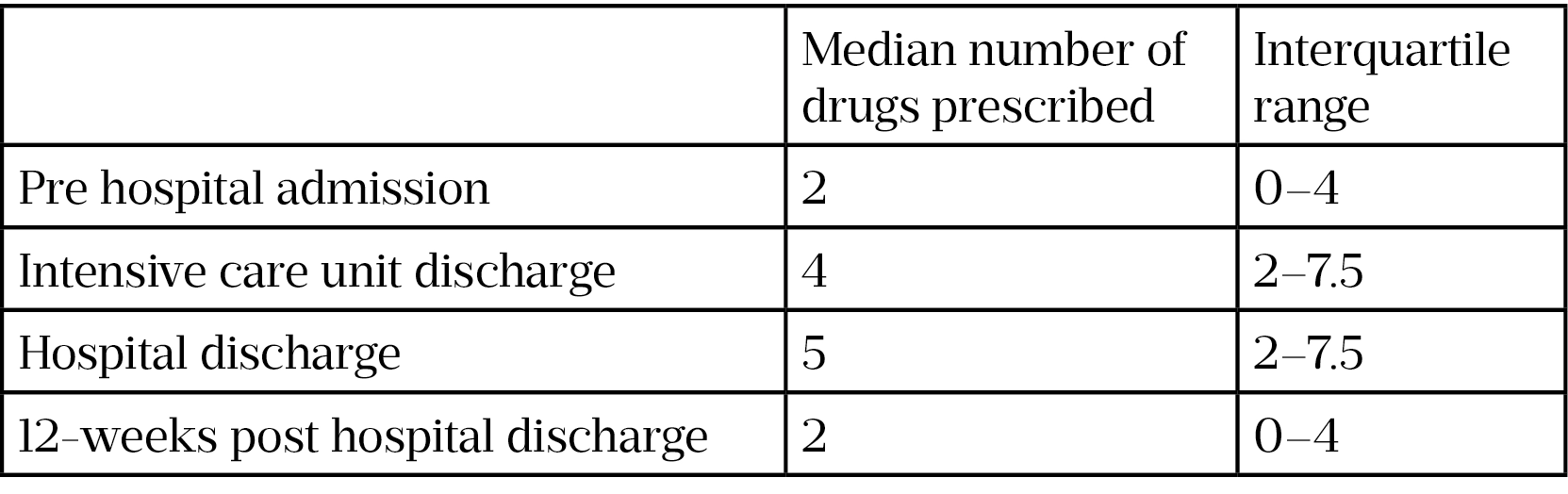
It was noted that there was a reduction in median number of drugs from discharge to 12 weeks post-discharge (i.e. 5 compared with 2). This can largely be attributed to cessation of short-term therapy (e.g. removal of thromboprophylaxis and antimicrobial medicines).
Medication-related interventions
Medication-related interventions were identified in 83% (n=32) of the patients who were involved with the service. The most common medication-related intervention was ‘patient education’, accounting for 38% (n=24) of the 64 identified (see Figure 1). This aimed to improve understanding of:
- Why and how to take the medication;
- The medical condition for which the medicines were being used;
- How to avoid side effects;
- A combination of the above.
This was followed by review of medication that was no longer appropriate on 16% (n=10) occasions.
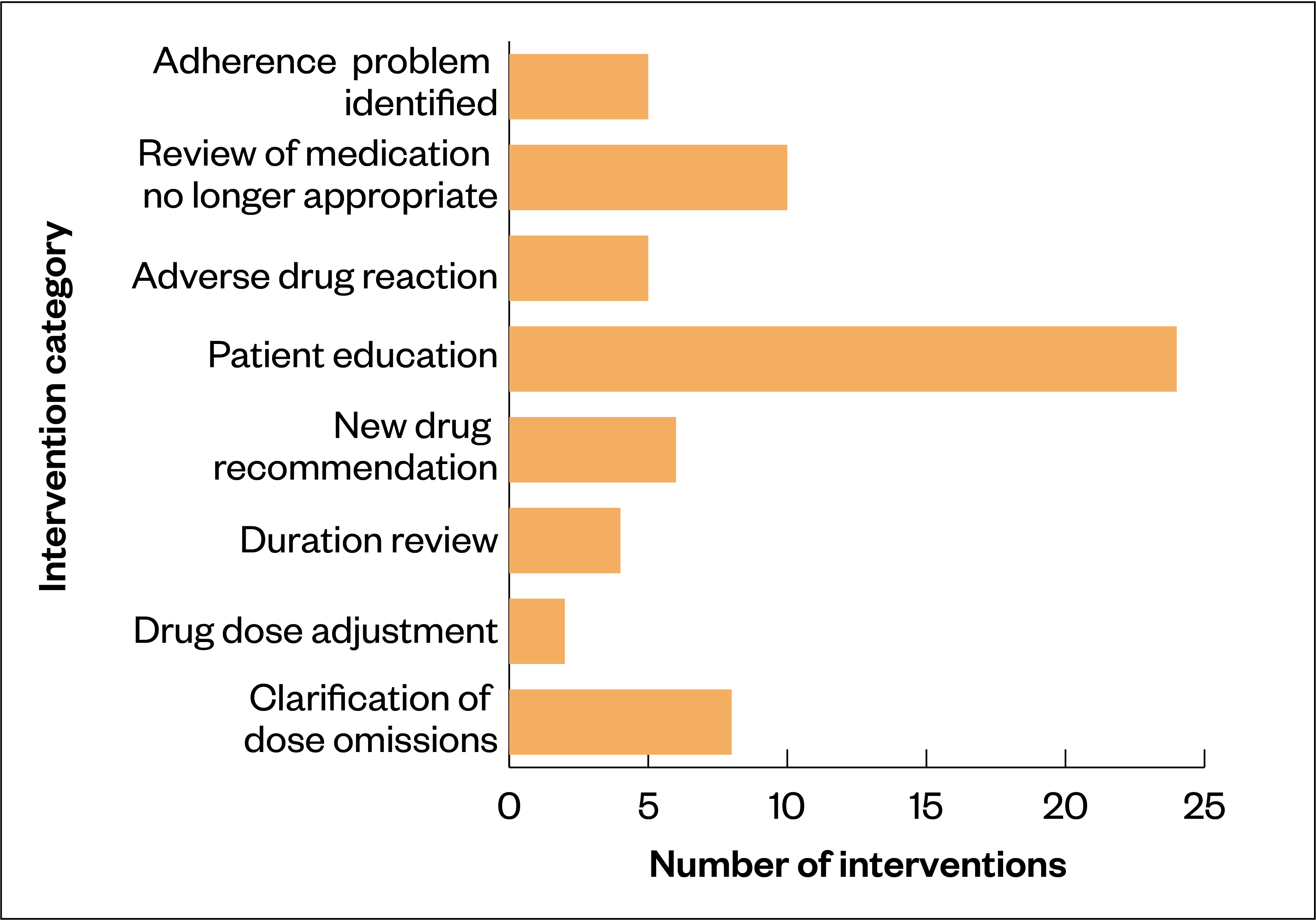
A total of 64 interventions were made by the clinical pharmacist for 39 patients, with 65% (n=42) of these being graded as Eadon ≥4 (i.e. significant interventions resulting in improved care standards). In comparison, for the same group of 39 patients, a total of 8 interventions were made at the 6-week clinic without clinical pharmacy input.
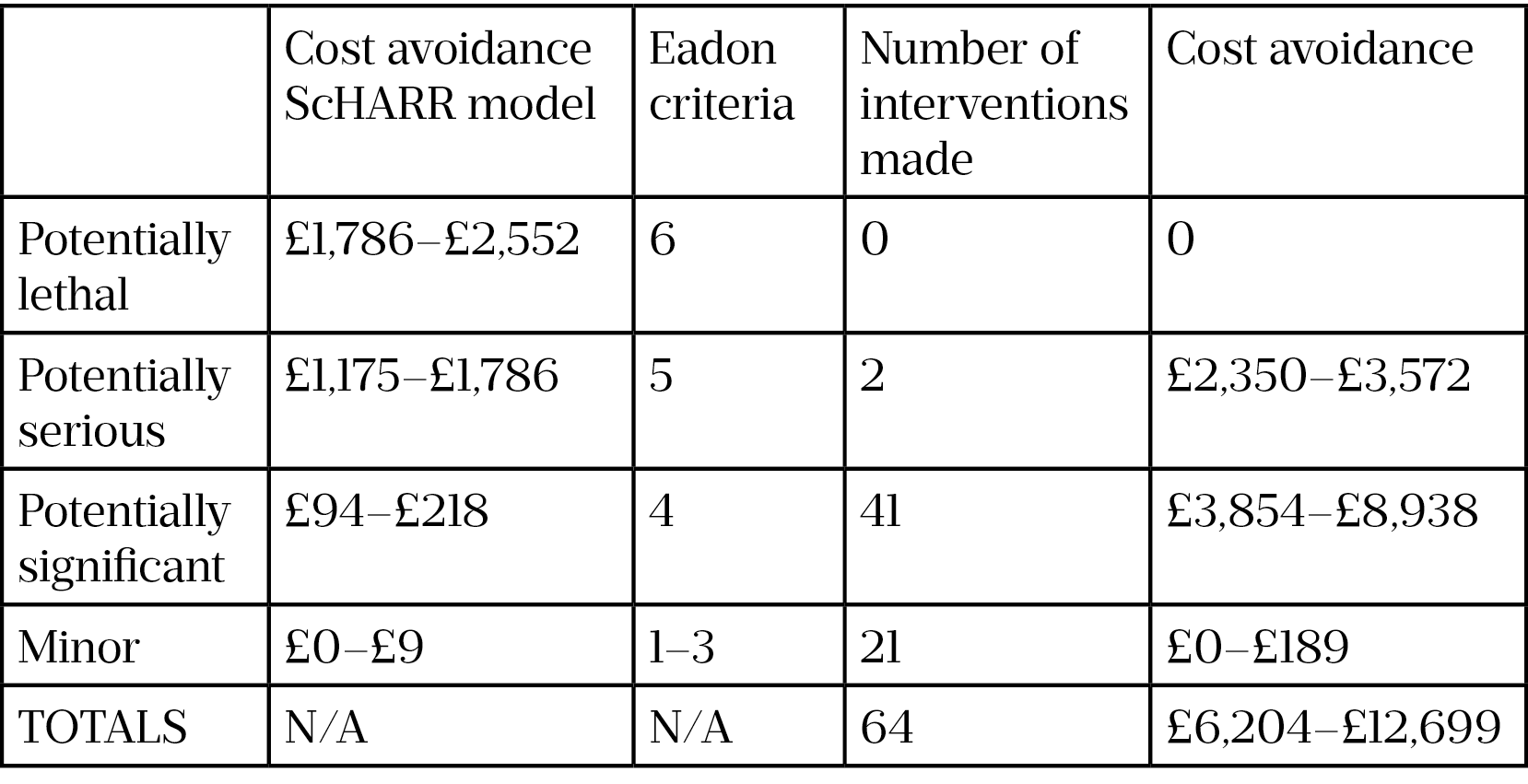
Examples of interventions included drug–herb interaction advice; discontinuing proton pump inhibitor therapy no longer required; inhaler technique counselling; highlighting key messages when non-adherence identified; and restarting a statin based on elevated lipid profile. Table 2 shows results from both the interventions made by the clinical pharmacist and the ScHARR model cost avoidance in terms of reducing healthcare resource utilisation. In addition, Karnon et al.‘s costs from 2006 were updated to reflect cost avoidance in 2020 by adjusting to the Retail Price Index[37].
Retail Price Index for 2006 = 202.7
Retail Price Index for 2020 = 294.2
Costs were revalued for 2020: sum of money x (294.2 ÷ 202.7)
An example of a grade 4 and a grade 5 intervention are shown in Table 3.
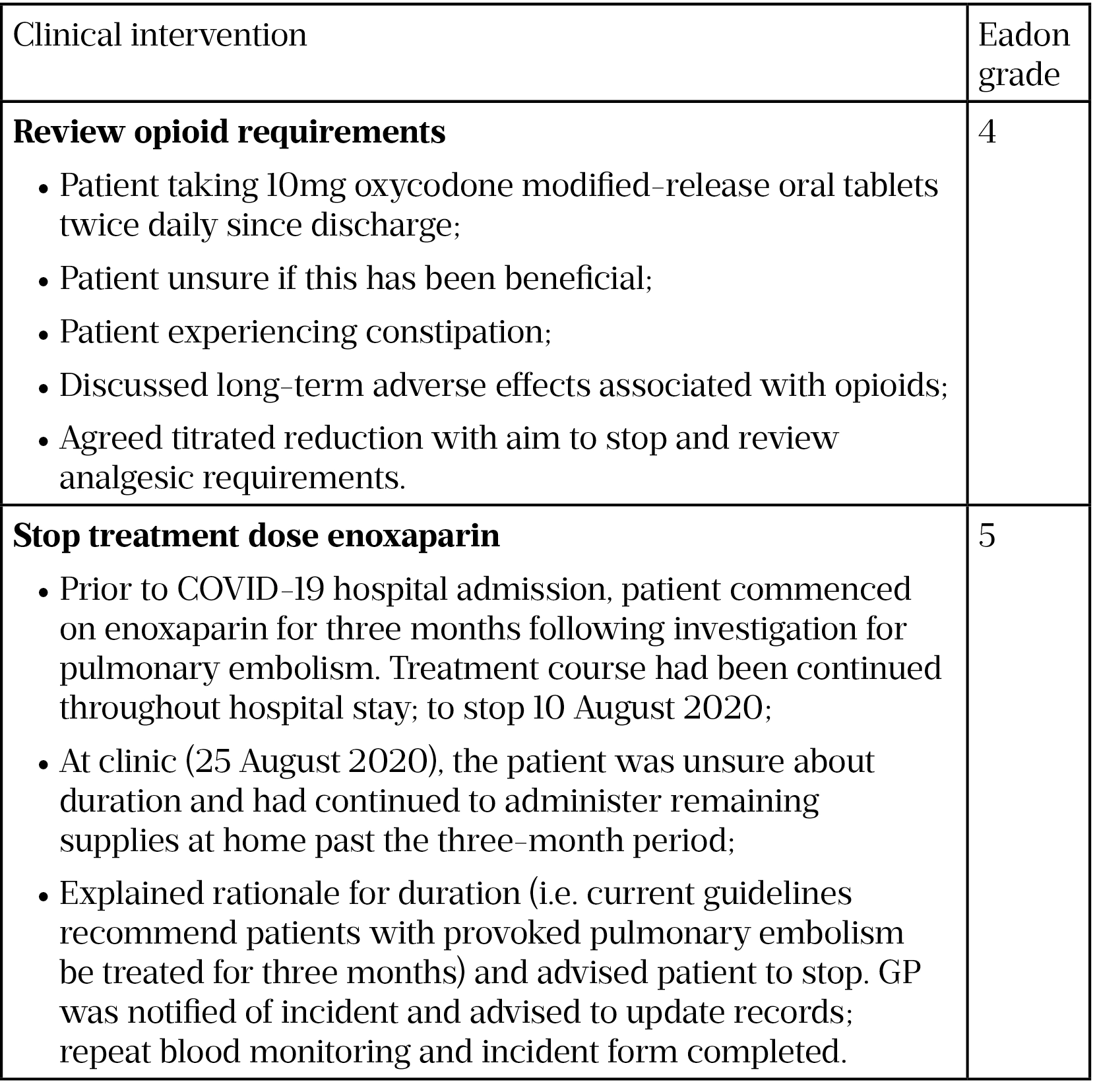
The total measurable potential savings in terms of reduction in healthcare resource utilisation and drug cost savings were estimated to be between £6,204 and £12,699, or £1,034–£2,116 per clinic day. The ‘invest to save’ return is the amount of potential savings based on every pound invested in the pharmacist’s participation in the clinic:
- Time taken for clinical pharmacist to contribute to 6 ICU follow-up clinics = 63 hours
- Cost for pharmacist participation (based on mid-point 8A) = £1,478
- Total measurable potential savings: between £6,204 and £12,699
- Invest to save return: between £6,204/1,478 and £12,699/1,478
This demonstrates an invest to save return in the range of £4.20–£8.59 per £1 invested.
Of the 64 interventions made, the classes of medication most associated with requiring an intervention were analgesics 30% (n=19) followed by cardiovascular 16% (n=10) (see Figure 2).
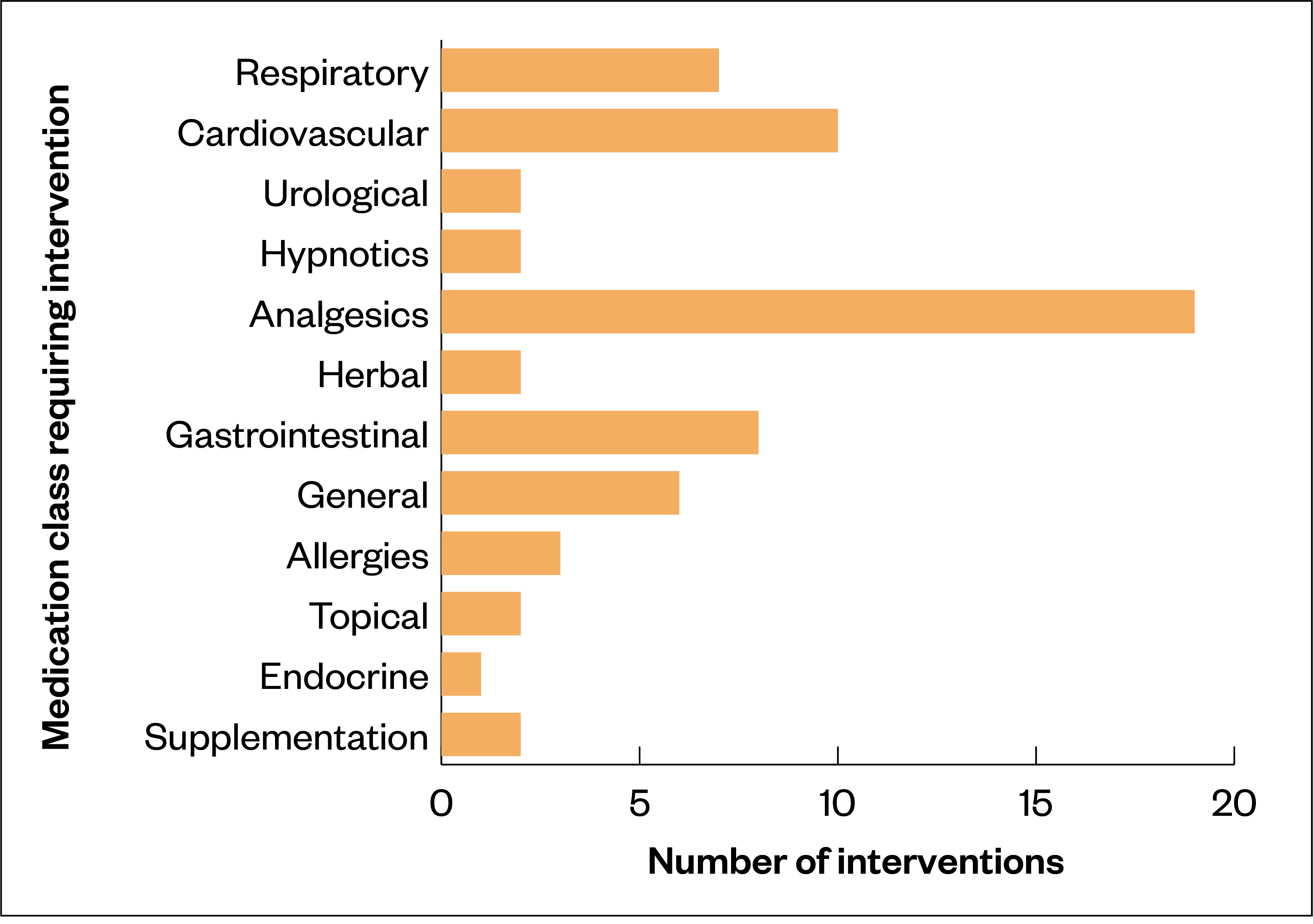
Patient feedback
Patients retrospectively completed a questionnaire assessing various aspects of their clinic visit, including how beneficial each health professional intervention was to their care. In total, 49% (n=19) of patients involved with the service responded to this questionnaire. All respondents rated the pharmacist intervention helpful, with 47% (n=9) indicating they benefited ‘a lot’ and 53% (n=10) indicating they received ‘some benefit’.
Discussion
In this study, it was observed that 82% (n=32) of patients required a medication intervention 12 weeks after discharge. This is consistent with previous studies in the post-ICU recovery setting. A 2018 study by Zahn et al. in the United States found that a critical care clinical pharmacist was able to provide an intervention at 81% (n=110) of patient visits to an ICU follow-up clinic, with a median of 1 (1, 2) intervention per visit[17]. Similarly, another US study by McDaniel et al. in 2019 found that medication-related interventions were provided by the clinical pharmacist for 75% (n=9) of patients attending a post-intensive care recovery clinic[18].
As seen in Figure 1, most interventions were related to symptom management and patient education. This reflects growing evidence that patients recovering from COVID-19 experience a high prevalence of physical, cognitive and psychological complications[38]. An online survey of doctors conducted by the British Medical Association in August 2020 found that around a third (n=1,092) said that they had seen or treated patients with symptoms they believed to be a long-term effect of the patient having had COVID-19[39]. The symptoms reported included chronic fatigue, muscle weakness, loss of sense of smell, and difficulties with concentration[39]. A team of researchers from Italy reported that 87% (n=125) of patients discharged from a Rome hospital after recovering from COVID-19 were still experiencing at least one symptom 60 days after onset. Of the 143 people discharged, 12.6% (n=18) were completely free of any symptoms, while 32% (n=46) had one or two symptoms, and 55% (n=79) had three or more symptoms[40].
Of the interventions undertaken, the majority were likely to improve patient care: 67% (n=43) of clinical pharmacist interventions were graded as Eadon ≥ grade 4 (i.e. significant interventions resulting in improved care standards). The ScHARR model highlights the potential cost savings of these interventions, with an invest to save return in the range of £4.20–£8.59 per £1 invested. There are limited studies evaluating the economic impact of pharmacist interventions in an outpatient setting. Chisholm et al. reported cost savings associated with a clinical pharmacist-managed medication assistance programme in a renal transplant clinic. For each US$1 spent in pharmacist’s time, a minimum of US$4 was returned to the institution[41].
In 2019, Al-Qudah et al. reported a benefit-to-cost ratio of 5.98 associated with clinical pharmacist interventions in a chronic disease outpatient setting[42].
Previous studies have used the ScHARR model to evaluate cost savings associated with pharmacist interventions across different settings. A retrospective, single-centre study in a 1,000-bedded teaching hospital in London set out to describe the cost benefits of pharmacy-led medicines reconciliation on admission. Using the ScHARR model, the 2012 study by Onatade et al. found the benefit:cost ratio was £5.53–£11.51 per £1 cost of employing a first-level post-foundation clinical pharmacist[43].
Ramsbottom et al. used the Eadon–Scharr model to assign clinical significance and monetary value to post-discharge interventions from community pharmacists. Their medicine review service for older people appeared to demonstrate a four-fold return on investment[44]. The same modelling was used to evaluate the impact of consultant pharmacist case management of older people in intermediate care[45]. Individualised pharmaceutical care plans were implemented for patients transferred to intermediate care. Clinical interventions were recorded and graded using Eadon criteria and cost savings resulting from interventions estimated using the ScHARR model. Miller et al. demonstrated an invest to save return in the range of £2.35–£4 per £1 invested[45].
The potential cost savings within the original ScHARR model are associated with subsequent healthcare utilisation, such as non-elective hospital admission, reduced bed days and outpatient attendances. The costs from 2006 can then be updated to reflect cost avoidance in 2020 by adjusting to the Retail Price Index. Pharmacist-led medicines optimisation clinics post-discharge can have a positive impact on medication adherence and health-related quality of life[46]. Further follow-up for COVID-19 patients discharged from hospital is required to assess if individual optimisation of medication has a long-term impact on improving patient outcomes for this patient cohort.
Previous studies within the post-ICU setting have mainly identified medication problems directly related to ICU interventions. A large population-based cohort study with 396,380 patients in Canada concluded that admission to an ICU was associated with an additional risk of medication discontinuation in 4 of 5 long-term medication groups vs hospitalisations without an ICU admission[47]. Similarly, a recent study in Scotland looking at pharmacist interventions at an ICU follow-up clinic found that the most common medication-related problem was omission of medicines for chronic disease states[16]. These medicines had been held during the patient’s hospital stay without being restarted. Unnecessary continuation of medicines has also been identified as a problem post-ICU. A prospective cohort study with 500 patients found that nearly half of all antipsychotic-naïve patients admitted with critical illness were treated with antipsychotics during their ICU stay, and one out of every four antipsychotic-treated patients was discharged on an antipsychotic even though the majority were no longer delirious[48].
Although medication choices in critical care were similar in this study, the inappropriate or unintentional changes associated with ICU intervention were less evident and only represented a small sample. There are several possible reasons that would require further investigation. This could be attributed to the low number of medications prescribed pre-admission (median of 2), hence there is proportionally less chance of inappropriate discontinuation of long-term therapy. In addition, it may also be explained by multiple opportunities for medication review prior to the 12-week clinic (i.e. step down to ward, hospital discharge and 6-week remote review).
Instead, a pattern of suboptimal medication therapy was found. Many interventions focused on improving the patients’ understanding of what medications were required for this stage of their recovery (e.g. appropriate use of different inhalers; formulation options for those with swallowing difficulties; and dose titration of laxatives). The nature of these interventions would also indicate that input into such a follow-up clinic required general clinical pharmacy skills rather than specialist critical care expertise. Reassurance around newly prescribed medication was an important aspect of the pharmacy consultation. Helping patients understand their medicines can help address anxiety related to PICS and COVID recovery. Although not covered by this research, this is important to consider, since those who have been seriously ill and hospitalised owing to COVID-19 are particularly vulnerable to mental health problems during the weeks and months of their recovery[25].
Analgesics was the class of drug most frequently associated with medication interventions 30% (n=19). This is unsurprising given those surviving critical illness with COVID-19 are at a risk of developing chronic pain[49]. It is likely this cohort will have undergone multiple interventions that are potentially painful, such as line insertion, turning, positioning and tracheal tube suctioning[50]. Considering the median length of ICU stay in this study was 12 days, these procedures will have been carried out on multiple occasions. Prolonged periods of immobilisation and ventilation can cause ICU-acquired weakness, which can manifest as chronic illness polyneuropathy[51].
Throughout the COVID-19 pandemic, proning (i.e. placing a ventilated patient on their front) has been a mainstay of ICU respiratory support to improve ventilation[52]. Complications associated with proning sedated patients (the process of turning a patient with precise, safe motions from their back onto their abdomen) include soft tissue and brachial plexopathy, potentially resulting in persistent neuropathic and musculoskeletal pain[53]. Emerging evidence from the pandemic suggests that COVID-19 itself can cause painful symptoms, including arthralgia, headache and myalgia[54]. Several case reports have shown that the virus also appears to have the capacity to induce painful para-infectious neurological disease such as Guillain–Barré syndrome and polyneuritis[55–58]. Within this study, the clinical pharmacist used their knowledge of the patient and available therapies to make recommendations on dose titration, choice and appropriate use of analgesics.
Utilising intensive care staff to provide this follow-up service has numerous benefits for patients as well as for staff. Familiarity with the patient’s ICU course helps the team make more appropriate decisions on therapy and they are well placed to understand, interpret and plan the recovery phase of the patients’ illness and signpost them to other hospital or community-based specialties[25]. Patient feedback at this stage can also influence changes to practice within the ICU. A qualitative inquiry via focus groups and interviews with ICU professionals in 2019 by Haines et al. found several mechanisms by which post-ICU care is perceived to improve care within ICU. Clinicians were able to reflect on their own practice by gaining greater insights into patient experience. Participants described how they were being able to recognise, anticipate and pre-empt patient and family needs post-ICU, during the ICU admission. The clinics offered the opportunity to close the feedback loop to ICU staff, highlighting positive outcomes of challenging cases and thus mitigating the risk of burnout for some clinicians[59].
Previous studies have shown that interventions from a clinical pharmacist in an ICU follow-up setting can identify and treat multiple medication-related problems as well as implement measures which prevent further adverse drug events[16–18,31,60]. Although this evaluation is set in the context of COVID-19, it adds weight to the benefits a critical care clinical pharmacist can deliver beyond patient discharge from ICU and the hospital setting.
Limitations
Although these results are representative of most patients discharged from COVID ICU during March–May 2020 in the Belfast Trust (42 referred to clinic and 39 attended), this was a local project involving only one centre. No comparison group of similar patients who were not seen by the clinical pharmacist at the clinic was readily available. In addition, some of the interventions required agreement and action from the patient’s GP or required the patient to adhere to advice given during the clinic visit. A further follow-up review would be required to assess if all recommendations were fully implemented
Ethics
As this study was a service evaluation and as all data were anonymised, ethical approval was confirmed to not be required, using the Health Research Authority assessment tool[61].
Implications for practice
In the UK, life after critical illness is an important work stream for the FICM[25]. It is hoped this study can contribute to data collection for service delivery and patient outcomes within this setting.
Within the Belfast Trust, the pilot has been granted approval to continue and the initial findings have been submitted to inform a regional strategy on COVID-19 rehabilitation and recovery services.
In our experience, most patients required a medication intervention and —with the associated potential cost savings — patients in this setting should therefore have access to a clinical pharmacist-led medication review. However, it is not clear at which stage of recovery this is most beneficial. Most of the medication interventions in this study would not have been apparent at discharge; however, all could potentially have been identified at the 6-week review. The 6- or 12-week period allows for short-term discharge therapy to be completed (e.g. antibiotics) and for existing symptoms to change (e.g. subside or develop further). Accurate medicines reconciliation for each distinct stage (e.g. pre-admission, post-ICU, discharge and post-discharge) is important before advising further on treatment plans. Clinical pharmacists are skilled to provide this service within the setting of a follow-up clinic and the invest to save results demonstrate an efficient use of available resources and also allows medical staff involved in the clinic to focus on other aspects of a holistic review.
Many of the interventions in this study were related to symptom management and are consistent with emerging evidence on COVID-19 complications, such as fatigue, dysphagia, cough, neuromuscular weakness, pain and neuropathy[25]. Healthcare professionals working within a post-ICU setting for COVID patients should be aware of long-term complications and the need to optimise symptom-related medication.
This study contributes to a body of evidence demonstrating the role of a clinical pharmacist in identifying and treating medication-related problems in an ICU recovery clinic. In addition, it provides information on the medication needs of those recovering from COVID-19 after a period in critical care. Our experience suggests that improving patients’ understanding of their medicines and optimising symptom-related medication are important aspects of COVID-19 recovery for those who required critical care treatment.
Conclusion
It is becoming increasingly clear that people who have been hospitalised, and subsequently recovered, from COVID-19 may need additional support with managing the long-term impact on their health. Initial experience from COVID-19 ICU recovery clinics suggest that significant physical, psychological and cognitive impairments may persist despite clinical resolution of the infection[62].
Based on the Institute of Public Care modelling for discharge pathways, the UK government predicts that 45% of hospitalised COVID-19 patients will need some form of low-level medical or social input for recovery and that 4% will require more focused, ongoing intense rehabilitation in a bedded setting[63,64]. Given the rising number of global infections, this suggests a cohort of critically ill survivors of unprecedented size. This emphasises the importance of post-ICU COVID-19 recovery and understanding the role of a clinical pharmacist within this setting.
In this study, we demonstrated medication interventions required for patients recovering from COVID-19 following a period in critical care. These interventions were associated with potential cost savings with an invest to save return in the range of £4.20–£8.59 per £1 invested. Our initial experience suggests an expanded role for a critical care clinical pharmacist within a MDT providing rehabilitation and recovery care for those affected by COVID-19. Further studies are required to both validate these results and evaluate long-term patient outcomes. In addition, studies could explore an appropriate pharmacy skill mix to support a post-COVID ICU pathway. Greater integration of pharmacy technician and pharmacist work streams in a critical care setting has the potential to enable the delivery of a more efficient clinical pharmacy service.
Financial and conflicts of interest disclosure
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was used in the production of this manuscript.
Acknowledgments
The authors thank all the patients involved in this evaluation and all staff in the Belfast Trust ICU rehab and recovery clinic.
References
- 1Adhikari NK, Fowler RA, Bhagwanjee S, et al. Critical care and the global burden of critical illness in adults. The Lancet 2010;376:1339–46. doi:10.1016/s0140-6736(10)60446-1
- 2Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit. Critical Care Medicine 2012;40:502–9. doi:10.1097/ccm.0b013e318232da75
- 3Harvey MA, Davidson JE. Postintensive Care Syndrome. Critical Care Medicine 2016;44:381–5. doi:10.1097/ccm.0000000000001531
- 4Marra A, Pandharipande PP, Girard TD, et al. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness*. Critical Care Medicine 2018;46:1393–401. doi:10.1097/ccm.0000000000003218
- 5Iwashyna TJ, Ely EW, Smith DM, et al. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA 2010;304:1787. doi:10.1001/jama.2010.1553
- 6Balas M, Buckingham R, Braley T, et al. Extending the ABCDE Bundle to the Post-Intensive Care Unit Setting. J Gerontol Nurs 2013;39:39–51. doi:10.3928/00989134-20130530-06
- 7Schweickert W, Kress J. Implementing early mobilization interventions in mechanically ventilated patients in the ICU. Chest 2011;140:1612–1617.https://doi.org/10.1378/chest.10-2829
- 8Peris A, Bonizzoli M, Iozzelli D, et al. Early intra-intensive care unit psychological intervention promotes recovery from post traumatic stress disorders, anxiety and depression symptoms in critically ill patients. Critical Care 2011;15:R41. doi:10.1186/cc10003
- 9Czerwonka AI, Herridge MS, Chan L, et al. Changing support needs of survivors of complex critical illness and their family caregivers across the care continuum: A qualitative pilot study of Towards RECOVER. Journal of Critical Care 2015;30:242–9. doi:10.1016/j.jcrc.2014.10.017
- 10Petrinec AB, Mazanec PM, Burant CJ, et al. Coping Strategies and Posttraumatic Stress Symptoms in Post-ICU Family Decision Makers*. Critical Care Medicine 2015;43:1205–12. doi:10.1097/ccm.0000000000000934
- 11Rehabilitation after critical illness in adults. Clinical guideline [CG83]. National Institute for Health and Care Excellence. 2009.https://www.nice.org.uk/guidance/cg83 (accessed Apr 2021).
- 12Connolly B, Douiri A, Steier J, et al. A UK survey of rehabilitation following critical illness: implementation of NICE Clinical Guidance 83 (CG83) following hospital discharge. BMJ Open 2014;4:e004963. doi:10.1136/bmjopen-2014-004963
- 13Rehabilitation after critical illness in adults. Quality standard [QS158]. National Institute for Health and Care Excellence. 2017.https://www.nice.org.uk/guidance/qs158/resources/rehabilitation-after-critical-illness-in-adults-pdf-75545546693317 (accessed Apr 2021).
- 14Goddard S, Cuthbertson B, Stevens R. ICU follow-up clinics. In: Textbook of post-ICU medicine: the legacy of critical care . Oxford: : Oxford University Press 2014. 603–612.
- 15Lasiter S, Oles SK, Mundell J, et al. Critical Care Follow-up Clinics. Clin Nurse Spec 2016;30:227–37. doi:10.1097/nur.0000000000000219
- 16MacTavish P, Quasim T, Shaw M, et al. Impact of a pharmacist intervention at an intensive care rehabilitation clinic. BMJ Open Qual 2019;8:e000580. doi:10.1136/bmjoq-2018-000580
- 17Zahn E, Fritschle-Hilliard A, Whitten J, et al. Role of a pharmacist in a critical care recovery clinic. Critical Care Medicine 2019;47:638. doi:10.1097/01.ccm.0000552068.47933.19
- 18McDaniel C, Brown J, Madara J, et al. Characterizing pharmacist interventions in an ICU recovery clinic. Critical Care Medicine 2020;48:380–380. doi:10.1097/01.ccm.0000631340.60380.e1
- 19Outbreak of pneumonia caused by new coronavirus. World Health Organisation. 2020.https://www.who.int/ith/2020-24-01-outbreak-of-Pneumonia-caused-by-new-coronavirus/en/ (accessed Apr 2021).
- 20WHO director general’s opening remarks at the media briefing on COVID-19 on 11th March 2020. World Health Organization. 2020.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (accessed Apr 2021).
- 21Gavriatopoulou M, Korompoki E, Fotiou D, et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med 2020;20:493–506. doi:10.1007/s10238-020-00648-x
- 22Lighter J, Phillips M, Hochman S, et al. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clinical Infectious Diseases 2020;71:896–7. doi:10.1093/cid/ciaa415
- 23Nandy K, Salunke A, Pathak SK, et al. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020;14:1017–25. doi:10.1016/j.dsx.2020.06.064
- 24Meng Y, Lu W, Guo E, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol 2020;13. doi:10.1186/s13045-020-00907-0
- 25Recovery and rehabilitation for patients following the pandemic. The Faculty of Intensive Care Medicine. 2020.https://www.ficm.ac.uk/sites/default/files/ficm_rehab_provisional_guidance.pdf (accessed Apr 2021).
- 26‘I could barely function’ – the devastating effects of long COVID. The Pharmaceutical Journal Published Online First: 2020. doi:10.1211/pj.2020.20208498
- 27COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE guideline (NG188) . National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ng188 (accessed Apr 2021).
- 28Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. 2020. doi:10.1101/2020.10.15.20205054
- 29PHOSP-COVID improving long term health outcomes. NIHR Leicester Biomedical Research Centre. 2020.https://www.phosp.org/ (accessed Apr 2021).
- 30Research and Innovation for post Covid-19 Rehabilitation (RICOVR). Sheffield Hallam University. 2020.https://www.shu.ac.uk/research/specialisms/advanced-wellbeing-research-centre/ricovr (accessed Apr 2021).
- 31MacTavish P. Medication-related problems in intensive care unit survivors: learning from a multicenter program. AnnalsATS 2020;17:1326–1329.https://doi.org/10.1513/AnnalsATS.202005-444RL
- 32Engström Å, Andersson S, Söderberg S. Re-visiting the ICU. Intensive and Critical Care Nursing 2008;24:233–41. doi:10.1016/j.iccn.2008.03.002
- 33Northern Ireland Electronic Care Record (NIECR). NI Direct Government services. 2013.https://www.nidirect.gov.uk/articles/northern-ireland-electronic-care-record-niecr (accessed Apr 2021).
- 34Pay bands in health & social care. Health and Social Care Jobs in Northern Ireland. 2019.https://jobs.hscni.net/Information/8/pay-bands-in-health-social-care (accessed Apr 2021).
- 35Eadon H. Assessing the quality of ward pharmacists’ interventions. International Journal of Pharmacy Practice 1992;1:145–7. doi:10.1111/j.2042-7174.1992.tb00556.x
- 36Karnon J, Mcintosh A, Dean J, et al. Modelling the Expected Net Benefits of Interventions to Reduce the Burden of Medication Errors. J Health Serv Res Policy 2008;13:85–91. doi:10.1258/jhsrp.2007.007011
- 37CPI and RPI Reference Tables, December 2020. Office for National Statistics. 2020.https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/chaw/mm23 (accessed Apr 2021).
- 38After-care needs of inpatients recovering from COVID-19. NHS England. 2020.https://www.cambscommunityservices.nhs.uk/docs/default-source/luton-adults-general/c0388_after_care_needs_of_inpatients_recovering_from_covid-19_5_june_2020.pdf (accessed Apr 2021).
- 39Rimmer A. Covid-19: Impact of long term symptoms will be profound, warns BMA. BMJ 2020;:m3218. doi:10.1136/bmj.m3218
- 40Carfì A, Bernabei R, Landi F, et al. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020;324:603. doi:10.1001/jama.2020.12603
- 41Chisholm MA, Vollenweider LJ, Mulloy LL, et al. Cost-benefit analysis of a clinical pharmacist-managed medication assistance program in a renal transplant clinic. Clin Transplant 2000;14:304–7. doi:10.1034/j.1399-0012.2000.140405.x
- 42Al‐Qudah RA, Al‐Badriyeh D, Al‐Ali FM, et al. Cost‐benefit analysis of clinical pharmacist intervention in preventing adverse drug events in the general chronic diseases outpatients. J Eval Clin Pract 2019;26:115–24. doi:10.1111/jep.13209
- 43Onatade R, Quaye S. Economic value of pharmacy-led medicines reconciliation at admission to hospital: an observational, UK-based study. Eur J Hosp Pharm 2016;25:26–31. doi:10.1136/ejhpharm-2016-001071
- 44Ramsbottom H, Rutter P, Fitzpatrick R. Post discharge medicines use review (dMUR) service for older patients: Cost-savings from community pharmacist interventions. Research in Social and Administrative Pharmacy 2018;14:203–6. doi:10.1016/j.sapharm.2017.02.007
- 45Miller E, Darcy C, Friel A. Consultant pharmacist case management of older people in intermediate care: a new innovative model. European Journal for Person Centered Healthcare 2016;4:1–7.http://bjll.org/index.php/ejpch/article/view/1056
- 46Odeh M, Scullin C, Hogg A, et al. A novel approach to medicines optimisation post-discharge from hospital: pharmacist-led medicines optimisation clinic. Int J Clin Pharm 2020;42:1036–49. doi:10.1007/s11096-020-01059-4
- 47Bell CM, Brener SS, Gunraj N, et al. Association of ICU or Hospital Admission With Unintentional Discontinuation of Medications for Chronic Diseases. JAMA 2011;306. doi:10.1001/jama.2011.1206
- 48Tomichek JE, Stollings JL, Pandharipande PP, et al. Antipsychotic prescribing patterns during and after critical illness: a prospective cohort study. Crit Care 2016;20. doi:10.1186/s13054-016-1557-1
- 49Kemp HI, Corner E, Colvin LA. Chronic pain after COVID-19: implications for rehabilitation. British Journal of Anaesthesia 2020;125:436–40. doi:10.1016/j.bja.2020.05.021
- 50Puntillo KA, Max A, Timsit J-F, et al. Determinants of Procedural Pain Intensity in the Intensive Care Unit: The Europain Study. Am J Respir Crit Care Med 2013;:131126070246006. doi:10.1164/rccm.201306-1174oc
- 51Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Critical Care Medicine 2011;39:371–9. doi:10.1097/ccm.0b013e3181fd66e5
- 52Clinical guide for the management of critical care for adults with COVID-19 during the coronavirus pandemic . National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/Media/Default/About/COVID-19/Specialty-guides/Specialty-guide_Adult-critical-care.pdf (accessed Jan 2021).
- 53Goettler CE, Pryor JP, Reilly PM. Crit Care 2002;6:540. doi:10.1186/cc1823
- 54Lovell N, Maddocks M, Etkind SN, et al. Characteristics, Symptom Management, and Outcomes of 101 Patients With COVID-19 Referred for Hospital Palliative Care. Journal of Pain and Symptom Management 2020;60:e77–81. doi:10.1016/j.jpainsymman.2020.04.015
- 55Padroni M, Mastrangelo V, Asioli GM, et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol 2020;267:1877–9. doi:10.1007/s00415-020-09849-6
- 56Chan M, Han SC, Kelly S, et al. A case series of Guillain-Barré Syndrome following Covid-19 infection in New York. Neurol Clin Pract 2020;:10.1212/CPJ.0000000000000880. doi:10.1212/cpj.0000000000000880
- 57Ebrahimzadeh SA, Ghoreishi A, Rahimian N. Guillain-Barré Syndrome associated with the coronavirus disease 2019 (COVID-19). Neurol Clin Pract 2020;:10.1212/CPJ.0000000000000879. doi:10.1212/cpj.0000000000000879
- 58Sancho-Saldaña A, Lambea-Gil Á, Liesa JLC, et al. Guillain–Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin Med 2020;20:e93–4. doi:10.7861/clinmed.2020-0213
- 59Haines KJ, Sevin CM, Hibbert E, et al. Key mechanisms by which post-ICU activities can improve in-ICU care: results of the international THRIVE collaboratives. Intensive Care Med 2019;45:939–47. doi:10.1007/s00134-019-05647-5
- 60Stollings JL, Bloom SL, Wang L, et al. Critical Care Pharmacists and Medication Management in an ICU Recovery Center. Ann Pharmacother 2018;52:713–23. doi:10.1177/1060028018759343
- 61Do I need NHS REC review? . NHS Health Research Authority. 2020.http://www.hra-decisiontools.org.uk/ethics/ (accessed Apr 2021).
- 62O’Brien H, Tracey MJ, Ottewill C, et al. An integrated multidisciplinary model of COVID-19 recovery care. Ir J Med Sci Published Online First: 7 September 2020. doi:10.1007/s11845-020-02354-9
- 63Reducing delays in hospital transfers of care for older people: key messages in planning and commissioning. 63. Institute of Public Care. 2018.https://ipc.brookes.ac.uk/publications/reducing-delays-in-hospital-transfers-of-care-for-older-people-key-messages-in-planning-and-commissioning (accessed Apr 2021).
- 64Hospital discharge service: policy and operating model . Department of Health and Social Care. 2020.https://www.gov.uk/government/publications/hospital-discharge-service-policy-and-operating-model/hospital-discharge-service-policy-and-operating-model (accessed Apr 2021).