In order to allow pharmacists to fulfil the ambitions laid out in four key policy papers about the future direction of development of pharmacy services,[1],[2],[3],[4]
changes in legislation and pharmacists’ roles were needed. Inherent in these was the need for pharmacists to work more flexibly in providing services. The new role outlined was that of the responsible pharmacist (RP).[5]
The RP would be the pharmacist responsible for securing the safe and effective running of the pharmacy and is a responsibility different from that of the superintendent pharmacist whose duties remain intact and are not negated by the RP.
The main duties of the RP are to:
- Secure the safe and effective running of the pharmacy, including during periods of absence
- Display a notice with the RP’s name, registration number and the fact that he or she is in charge of the pharmacy at that time
- Complete the pharmacy record to identify who the RP is for a pharmacy at any one time
- Establish (if not already established), maintain and keep under review procedures for safe working
Crucially, the RP is allowed to be absent from the premises for a maximum of two hours in any 24-hour period (midnight to midnight) to provide pharmaceutical services in a different setting. To facilitate this, the Health Act 2006 amended the relevant section of the Medicines Act 1968 pertaining to “personal control” and the Medicines (Pharmacies) (Responsible Pharmacist) Regulations 2008 introduced to set out the duties of RPs. The legislative change is important in that, now enshrined in law, any deviance or omission arising in duties set out, may have legal consequences.
The Medicines Regulations 2008 made further amendments to allow the sale of general sale list medicines in the absence of the RP. The sale or supply of prescription-only and pharmacy medicines in the absence of the RP is still curtailed.
The Government undertook a 13-week consultation exercise from 24 October 2007,5 the responses to which are available to view.[6]
The Department of Health indicated that it had worked with a number of pharmacy organisations and pharmacy journals to highlight this consultation. The then professional regulator, the Royal Pharmaceutical Society of Great Britain, also held a consultation on its professional standards and guidance document in order to obtain input from members on its proposed standards for RPs. These standards aimed to assist members to comply with the RP Regulations. The consultation was made available via the Society’s website as well as through The Pharmaceutical Journal of 17 January 2009.[7]
The consultation had a closing date of 6 March 2009. Members were encouraged to respond via the website or by posting in the questionnaire provided in The Journal.
The RPSGB distributed the “Responsible pharmacist toolkit” to all registered pharmacists to clarify the role in May 2009.[8]
The toolkit was an educational booklet that aimed to clarify the new role and associated Regulations by illustrations with case studies and scenarios and a “test your knowledge” section to work through.
During the study period, the Chemist and Druggist community pharmacy publication covered the new role and associated Regulations extensively in articles and opinion pieces from commentators such as the pharmacist Sandra Gidley (who was then a Liberal Democrat MP and shadow health spokeswoman).[9]
LocumVoice[10]
and the Pharmacists’ Defence Association.[10],[11],[12],[13],[14]
Interestinglythe only exclusively positive comments came from the DoH and there were some mixed views that recognised the opportunities afforded by the new role but expressed reservations about the legal implications.[15]
Most of the press coverage was negative, ranging from the doomsday scenario of the “beginning of the end for the pharmacy profession”[16]
to highlighting concerns with the discriminatory element vis-à-vis European qualified pharmacists,[17]
rest breaks, following of standard operating procedures and a plethora of legal concerns.[9],[10],[11],[12],[13],[14],[15],[16],[17],[18]
The Royal Pharmaceutical Society (RPS), and the Professional Forum of the Pharmaceutical Society of Northern Ireland (PSNI) have now commissioned research into the impact of the RP Regulations on pharmacy practice in the UK.[19]
The evaluation described here, undertaken in 2009, provides context regarding the views of pharmacists before the new regulations were introduced.
The aim of this project was to investigate the views of non-contractor community pharmacists regarding the new RP role and regulations before the implementation of this new legislation, in particular to:
- Determine if community pharmacists viewed the RP Regulations and role as a positive development
- Ascertain if community pharmacists intended to change their future career and/or work pattern in response to the introduction of the RP Regulations and role
- Explore whether pharmacists’ views were associated with demographic factors
Methods
The project design was a cross-sectional, anonymous self-completion postal survey. The study period was summer 2009, just before the implementation of the RP Regulations on 1 October 2009.
Employee and locum pharmacists working in community pharmacies within the PCTs surrounding the University of Central Lancashire formed the study sample frame (NHS Central Lancashire, East Lancashire, North Lancashire, Blackpool, and Blackburn with Darwen). There were 335 pharmacies in this area, according to the address lists from the primary care trusts.
The UCLan school of pharmacy’s internal ethics committee gave its approval on 6 June 2009. The local research ethics committee indicated that its approval was not required for this type of evaluation.
The survey was compiled by an in-house team, and was reviewed by the professional development manager of Central Lancashire Local Pharmaceutical Committee and by the regional Pharmaceutical Services Negotiating Committee representative for face validity. A pilot was conducted among a small number of pharmacies in a different geographical area.
The self-completion survey included the questions in Panel 1. Participants were given the choice of indicating their view by ticking “yes”, “no” or “not sure”. Space was given below each question for participants to comment on their response.This paper is focused on the responses to questions 1 (“do you think the responsible pharmacist role is a positive development?”) and 2 (“do you think the changes to your role will affect your future career and/or work pattern?”).
Panel 1: Survey questions
- Do you think the responsible pharmacist role is a positive development?
- Do you think the changes to your role will affect your future career and/or work pattern?
- Have you taken any steps to prepare for your new role?
- Has your employer(s) taken any steps to prepare for your new role?
- You will be allowed to leave the pharmacy for a max of two hours in any 24 in order to allow you to provide additional services outside. What are your views on this?
- As a pesponsible pharmacist you will have to secure the safe and effective running of the pharmacy (including during periods of absence) and establish (if not already established), maintain and keep under review procedures for safe working. What are your views on this?
- The pharmacist who is the responsible pharmacist will, for the first time, have statutory duties, breaches of which could lead to them being prosecuted. What are your views on this?
- Are there any other issues not covered above that you would like to share?
Data collected were: gender; whether the pharmacist was employed or a locum; number of years qualified as a pharmacist; whether the pharmacist had participated in any of the consultations that had taken place regarding the new Regulations; and if the pharmacist had fully read the RPSGB RP toolkit. The survey was distributed by post at the beginning of July 2009, with two follow-up reminders. Data collection finished at the beginning of September.
Quantitative survey responses were entered into a PASW database.[20]
Descriptive frequencies and the chi-squared comparative statistical test were performed as appropriate for the data. Qualitative comments associated with questions 1 and 2 were subjected to thematic analysis. SV did the first round of coding for both questions, and NG independently coded a subset of the comments for question 1.
Results and discussion
Of the 335 questionnaires distributed, 142 were returned (42 per cent). Of these, 26 were from contractors. Contractors did not form part of the study, and were therefore asked not to complete the questionnaires but to return them anyway to prevent unnecessary follow-up. These were removed from the study sample. One questionnaire was returned blank, without any indication whether the respondent was a contractor. Therefore the number of returned questionnaires used for evaluation was 116. This gave a usable response rate of 35 per cent (116/335). Descriptive data about the respondents are given in Table 1.
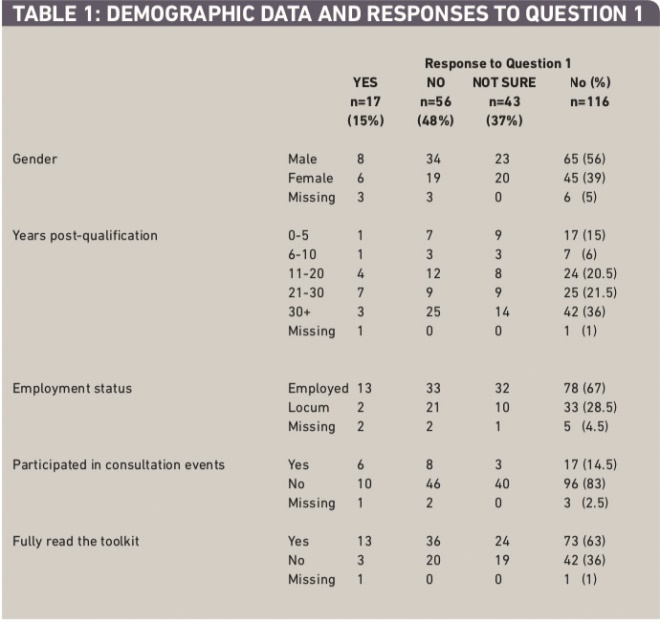
Table 1: Demographic data and responses to question 1
In the pharmacy workforce census data 2008,[21]
the female:male ratio of pharmacists was 3:2. In the present study it was 2:3, so male pharmacists were over-represented in the sample. In the census data, 65.5 per cent of pharmacists were under 50 years old. The respondents for this study finding were similar at 63 per cent (0–30 years post-qualification, assuming that most respondents qualified around the age of 21).
Quantitative survey data
Only 15 per cent of respondents viewed the new role and regulations as being a positive development for pharmacy. Almost half of all respondents (48 per cent) did not think the new regulations and role were a positive development. The remaining respondents (37 per cent) indicated that they were unsure about the role.
Almost two-thirds of respondents had fully read the RP toolkit (63.5 per cent), but only a minority (15 per cent) had participated in any of the consultation events.
For each of the independent demographic and role variables shown in Table 1, a chi-squared test was conducted to explore their association with the responses to question 1 (“Do you think the responsible pharmacist role is a positive development?”), excluding missing responses. The only independent variable approaching a significant association with the response to question 1 was participation in consultation events (c2=8.505, P <0.05), although the result validity was limited by the small number of responses. A positive response to question 1 was associated with having participated in a consultation event.
Respondents gave mixed responses to whether the RP development would change their future career or work pattern: 37 per cent indicated “yes”, 37 per cent indicated “no”, and the remaining 25 per cent were “unsure” (one response missing). The nature of any intended change is further discussed in the next section about the open comments.
Qualitative open comments
Most of the returned survey forms included at least one written comment from the respondent, and a significant number included lengthy comments about each question.Tables 2–5 contain an overview of open comments related to each question: they may not be considered representative due to low numbers, but provide an interesting overview of a range of concerns and perspectives.
An increase in accountability and empowerment was cited as the main reason for the new regulations and role being a positive development (Table 2). Nine out of these 11 positive respondents were employees. Many of them thought that the regulations would force locums to become more accountable. As one respondent said: “Hopefully [this] will make locums think more about what they are doing.”

Table 2: Positive comments
The greatest concern of respondents was the perceived detrimental impact of the regulations on working life with regards to workload and stress, liability, and potential for confusion (Table 3). Locum comments on this issue included the perceived increase in liability, such as “more susceptibility to prosecution or disciplinary hearings”. Other locums asserted that the new regulations were an attempt to transfer responsibility, accountability and liability (RAL) from employers to employees and that the new regulations were a “get-out clause for companies”, “an excellent blame tool”, making employers “safe from prosecution”.
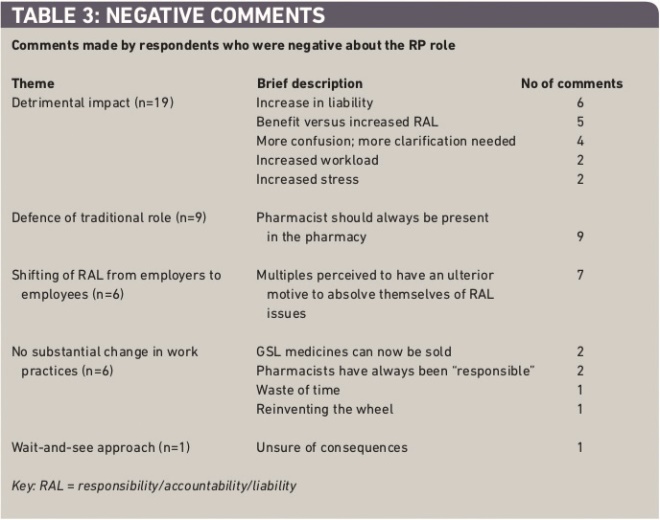
Table 3: Negative comments
Doubts were also raised as to whether any stated benefit of the new regulations outweighed the increase in RAL associated with the new regulations — “new role offers little advantage to pharmacists but requires more responsibility from already pushed community pharmacists” and “too much responsibility and not enough to benefit us”. Many pharmacists still thought that pharmacists should always be present on the premises during opening hours, as it was “impossible to be in control of daily activities when you are not at the pharmacy” and “we are the only health care professional the public can access without an appointment. We should continue to be present at all opening times.”
There was a perception that the regulations were geared primarily in favour of the multiples which wanted to transfer RAL from senior head office staff to individual pharmacists in the pharmacies. Examples of comments include “it is shifting the responsibilities of contractors &/or superintendents on to the pharmacists, without providing the autonomy or power and support in their working environment” and “the pharmacist is now the scapegoat with more liability, ie, like the superintendent without the money and more stress”. A few respondents viewed the regulations negatively because the regulations would not substantially change day-to-day working practices evidenced from comments such as “effectively there is no change”, “doesn’t really create any ground breaking opportunities”, and “difficult to see how it changes practice day-to-day”.
Most of those who identified themselves as “unsure” had adopted a “wait and see” approach (Table 4). Only this cohort of respondents voiced concerns regarding the two-hour absence that would be allowed, and the responsibilities ensuing from this, with one respondent stating “pharmacists will be reluctant to leave the premises especially with the recent Elizabeth Lee case”. They were also the only ones to indicate that they believed the regulations were rushed through without a thorough understanding of their consequences, with pharmacists indicating that they think there are “gaps” in the regulations, that the regulations were “half hearted half thought out” and how it all played out depended on “how the regulations are interpreted by the courts”.
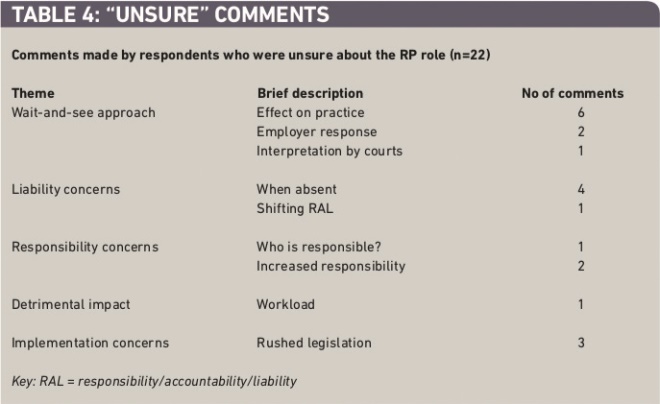
Table 4: ‘Unsure’ comments
Some gender effects in written comments were seen among the Question 1 negative response group. Both male and female respondents cited the perceived detrimental impact to their working lives as the reason for their stance. Women were most concerned, however, about the lack of benefit to them, set against the increase in RAL, with an increase in workload coming second. No concern for any increase in liability arising from the RP regulations was stated, yet this was the single largest cause for concern for the male cohort followed by increase in confusion surrounding who would actually be responsible given certain scenarios. Impact on workload was not mentioned by the men at all.
Description of planned changes to career and/or work patterns in Question 2 were provided by 16 respondents (Table 5). They described a number of different planned changes that they might make, including leaving the pharmacy profession and moving towards fewer, trusted places of work.

Table 5: Effects on future career or work
Implications for engagement of pharmacists with practice change
Reading the RP toolkit was not associated with a positive response to the role change: readers reflected a persisting mix of views and concerns. If its purpose was to allay any fears that pharmacists might have had regarding the introduction of the new regulations, its success was questionable. It might appear that the toolkit was characterised as a booklet of “do’s and don’ts”. There was no mention within the toolkit of any potential impact on individual pharmacists’ day-to-day work or, indeed, of such emotive issues such as affect on workload, employer relations and remuneration. Yet these were the issues that were cited at length by our respondents. Furthermore the toolkit fully detailed new statutory responsibilities pertaining to the new RP role, and this in itself may have led to the overwhelming negative response. The challenge is thus to produce materials that provide factual information and an appreciation of the emotional and practical perspectives that pharmacists carry with them. Vignettes and case studies might be useful as illustrations about “pharmacists like me”.
Engagement with the consultation was associated with a more positive view. This might simply be because pharmacists attending such consultations are, by nature, enthused and open to new ideas — the “early adopters”. Since only one in seven respondents reported taking part in any of the consultation events pertaining to the new regulations, the question must be asked as to why most of them did not do so — considering the depth of negative feeling, uncertainty, misgivings and reservations expressed by respondents in this study. The evaluation of another major change in community pharmacy practice — the implementation of the new contract in 2005 — suggested an impact of disengagement, connected to low feelings of power and influence, on pharmacists’ negative perceptions of the changes.[22]
Limitations of the study
It was disappointing that the response rate was relatively low, as this was an opportunity for employee and locum pharmacists to engage with an imminent and fundamental change to everyday community pharmacy practice. There are, however, several reasons which might have affected the response rate.
The survey was posted to pharmacies, not individual pharmacists. This probably reduced the response rate. Since many large multiples prefer to see surveys before they are sent to employees, there may have been some resistance among employees to respond if they were unsure of the position of their employer.
The survey was undertaken in the summer months. This is generally the time when most employed pharmacists are on holiday and locums provide cover for this. Locums may have been less inclined to participate as they are only there temporarily and perhaps do not open the post.
During the review phase, the decision was made by the in-house team to change the research instrument from a primarily closed question format (using Likert scales to indicate strength of agreement or disagreement) to an open question format in order to ensure that questions did not direct participants to focus on a particular answer. This may have had an impact on responses because the questionnaire took longer to complete and the pharmacists had to consider their responses rather than “tick” a given list of options.
We were not able to able to follow up non-responders and we recognise that this will limit the generalisability of the survey. Although some of the questions in the survey were hypothetical these were necessary since the survey concerned the perception pharmacists had before implementation of the responsible pharmacist regulations. Respondents’ views may have changed post-implementation, and it was intended that this study would form a baseline for further investigation in the future once the regulations had been in embedded in practice.
Conclusion
Responses from this study show that almost six out of seven pharmacists who replied were either negative or uncertain about the new regulations and responsible pharmacist role. Pharmacists gave a variety of reasons for their responses. This reflected a breadth and depth of concern that pharmacists had in the period before implementation. The pharmacy press was awash with comments and articles with regard to the new regulations and RP role in the months leading up to the implementation date, and many of these also reflected negative views and concerns. The only significant statistical association was that pharmacists who had participated in consultations were more likely to express a positive view of the new role. This suggests that engagement was an important factor in dealing with the uncertainties of the pre-implementation period. Pharmacy policymakers must make more strenuous efforts to ensure that grassroots pharmacists are more fully engaged with professional developments which impact them and that they are given more opportunity to have their views and opinions heard on issues concerning them. The new research programme will explore how pharmacists have experienced this change in practice.
Acknowledgements
We thank the pharmacists who took part in the survey. We also thank the Pharmacy Practice group at the school of Pharmacy and Biomedical sciences at UCLan for their early assistance with the project design. We are grateful to Raymond Lee and Mark Collins for their review of the materials.
About the authors
Samir Vohra, BPharm, MRPharmS, is lecturer in clinical pharmacy and Nicola Gray, PhD, MRPharmS, is reader in medicines and health at the University of Central Lancashire School of Pharmacy and Biomedical Sciences.
Correspondence to: Samir Vohra (email svohra1@uclan.ac.uk)
References
[1] National Assembly for Wales. Remedies for success: a strategy for pharmacy in Wales. Cardiff: National Assembly for Wales; 2002.
[2] The Scottish Executive. The right medicine: a strategy for pharmaceutical care in Scotland. Edinburgh: The Scottish Executive; 2002.
[3] Department of Health (England). A vision for pharmacy in the new NHS. London: Stationery Office; 2003.
[4] DHSSPS. Making it Better: a strategy for pharmacy in the community. Belfast: DHSSPS; 2003.
[5] Department of Health (England). The responsible pharmacist: consultation on proposals for the content of the responsible pharmacist regulations. London: Stationery Office; 2008.
[6] Department of Health (England). The responsible pharmacist regulations: a summary of the responses to public consultation on proposals for the content of the regulations. London: Stationery Office; 2009.
[7] Society seeks members’ views on responsible pharmacist guidance. Pharmaceutical Journal 2009;282:35.
[8] The Royal Pharmaceutical Society of Great Britain. The responsible pharmacist toolkit. London: RPSGB; 2009.
[9] Pharmacist in the House: Who’s responsible for his confusion?Chemist and Druggist [online]. 27 August 2009. Available at: www.chemistanddruggist.co.uk (accessed 20 July 2011).
[10] Locums contact DH over responsible pharmacist rules.Chemist and Druggist [online]. 11 June 2009. Available at: www.chemistanddruggist.co.uk (accessed 20 July 2011).
[11] The risks of the responsible pharmacist. Chemist and Druggist [online]. 3 September original paper 2009. Available at: www.chemistanddruggist.co.uk (accessed 20 July 2011).
[12] Richardson J. Ministers rule out responsible pharmacist delay. Chemist and Druggist [online]. 9 September 2009. Available at: www.chemistanddruggist.co.uk (accessed 20 July 2011).
[13] Walkout threats over responsible pharmacist regulations. Chemist and Druggist [online]. 23 July 2009. Available at: www.chemistand druggist.co.uk (accessed 20 July 2011).
[14] Campaign to delay responsible pharmacist gathers pace. Chemist and Druggist [online]. 17 July 2009. Available at: www.chemistand druggist.co.uk (accessed 20 July 2011).
[15] The future of the responsible pharmacist. Chemist and Druggist [online]. 25 October 2007. Available at: www.chemistand druggist.co.uk (accessed 20 July 2011).
[16] Le Provost G. Responsible pharmacist under fire from industry. Chemist and Druggist [online]. 17 January 2008. Available at: www.chemistanddruggist.co.uk (accessed 20 July 2011).
[17] French locum: responsible pharmacist rules are discriminatory. Chemist and Druggist [online]. 1 October 2009. Available at: www.chemistanddruggist.co.uk (accessed 20 July 2011).
[18] Accountability unclear as RPSGB launches responsible pharmacist toolkit. Chemist and Druggist [online]. 30 April 2009. Available at: www.chemistanddruggist.co.uk (accessed 20 July 2011).
[19] Impact of RP — UK research launched. Pharmaceutical Journal 2011;287:42.
[20] PASW Statistics, Release Version 18.0.0 (SPSS, Inc., 2009, Chicago, IL, www.spss.com)
[21] Seston E, Hassell K. An overview of the main findings from the 2008 pharmacy workforce census. Pharmaceutical Journal 2009;283:419–20.
[22] Bond C, Blenkinsopp A, Inch J, Celino G, Gray N. The effect of the new community pharmacy contract on the community pharmacy workforce. London: Pharmacy Practice Research Trust; 2008.


