Source: Courtesy of Ashifa Trivedi
Ongoing trials to find effective COIVD-19 treatments include medicines that pharmacists are likely to encounter in practice
Key points
- Several medicines are undergoing investigation through clinical trials as potential treatments for COVID-19, but at this time none are licensed.
- The ‘Randomised Evaluation of COVID-19 Therapy trial’ (RECOVERY) commenced in March 2020 and includes patients with clinically suspected or laboratory-confirmed COVID-19 in hospital. Treatments currently included in this trial are: lopinavir-ritonavir; corticosteroids (paediatrics only); azithromycin, tocilizumab and convalescent plasma.
- Although many trials are ongoing, the numbers of patients involved are small and many of the studies have limitations.
- Pharmacists involved in these trials should consider how these medicines can impact patients with existing conditions or who are on other medicines, and provide advice on potential drug interactions, monitoring and adverse effects. The route of drug administration is an important consideration (e.g. lopinavir-ritonavir tablets cannot be crushed for administration via nasogastric tubes).
- Pharmacists should be aware that the doses being used in clinical trials may be different from the licensed doses of these medicines and, therefore, side effects may present differently.
- Dexamethasone is the first drug to be shown to improve survival in COVID-19.
Introduction
A novel coronavirus, designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first detected in December 2019 in Wuhan, China. It is a new strain of coronavirus, which causes an infection commonly referred to as COVID-19 that has not been previously identified in humans[1]
. Global research efforts into potential treatments started in January 2020 and there are now thousands of studies looking at how to treat and manage the disease. While there are no antivirals licensed for use for this indication, data from other infectious coronaviruses, including severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), as well as in vitro studies, demonstrate that there are potential benefits that could be obtained from antiviral therapy[2],[3]
.
On 20 March 2020, the World Health Organization (WHO) announced ‘Solidarity’, a large global trial that compares local standards of care with local standards of care plus one of the following treatments: remdesivir; hydroxychloroquine; lopinavir-ritonavir; or an arm that combines ritonavir-lopinavir with interferon-ß-1a. Adults with COVID-19 admitted to participant hospitals can join this study[4]
. As of 3 June 2020, around 3,500 patients have been enrolled from 35 countries[4]
.
In the UK, the ‘Randomised Evaluation of COVID-19 Therapy trial’ (RECOVERY) has recruited more than 11,748 patients from 176 NHS hospitals[5]
. Version 6.0 of the study protocol outlines how its factorial design ensures that eligible and consenting participants may be randomised to one of the treatment arms in Randomisation A and, simultaneously, to one of the treatment arms in Randomisation B (see Box)[5],[6],[7],[8]
.
This rapid review aims to summarise interventional trials, alongside in vitro studies, and examine several potential COVID-19 therapies to provide an insight into the global response to the COVID-19 pandemic. In addition, this rapid review considers what medicinal therapies pharmacists are most likely to encounter in practice, as a result of clinical trials, and the safety and practice considerations for these investigational medicines.
Box: Treatment arms included in the UK ‘Randomised evaluation of COVID-19 therapy’ (RECOVERY) trial
The RECOVERY trial is currently open for patient recruitment. To be eligible for the study, adult patients need to be in hospital; have confirmed or suspected COVID-19 (i.e. clinically suspected or laboratory-confirmed); and have no medical history that may, in the opinion of the attending clinician, put the patient at significant risk if they were to participate in the trial[5]
.
Randomisation A consists of five treatment arms:
- No additional treatment;
- Lopinavir–ritonavir 100mg by mouth (or nasogastric tube) every 12 hours for 10 days;
- Low-dose corticosteroids — in the form of dexamethasone administered orally (liquid or tablets) or intravenous preparation 6mg once-daily for 10 days. In women who are pregnant or breastfeeding, prednisolone 40mg administered by mouth (or intravenous hydrocortisone 80mg twice-daily) should be used instead of dexamethasone (this arm closed for enrolment on 8 June 2020, but is still recruiting for paediatric patients);
- Hydroxychloroquine should be given orally for a total of 10 days; the dosing is as follows: 800mg initially, 800mg 6 hours after the initial dose, 400mg 12 hours after the initial dose, 400mg 24 hours after the initial dose, then 400mg every 12 hours thereafter for 9 days (this arm closed for enrolment on 5 June 2020);
- Azithromycin 500mg orally (or via nasogastric tube) or intravenously once-daily for 10 days)[5]
.
The doses listed above are those that would be used for standard adult treatment. For information on paediatric doses, please refer to the RECOVERY trial protocol[5]
. On 5 June 2020, hydroxychloroquine was discontinued as a trial arm and the randomisation program has been amended so that no further participants will be allocated to hydroxychloroquine[6],[7]
. In response to a request by the Medicines and Healthcare products Regulatory Agency, the trial investigators reviewed the unblinded data on the hydroxychloroquine arm of the trial[7]
.
A total of 1,542 patients were randomised to hydroxychloroquine and compared with 3,132 patients randomised to usual care alone. The investigators found that there was no significant difference in the primary endpoint of 28-day mortality (25.7% hydroxychloroquine vs. 23.5% usual care; hazard ratio 1.11 [95% confidence interval 0.98-1.26]; P =0.10). There was also no evidence of beneficial effects on hospital stay duration or other outcomes[6],[7]
. It has therefore been recommended that any participants assigned hydroxychloroquine should discontinue the treatment.
Randomisation B involves receiving either no additional treatment or convalescent plasma. Participants with progressive COVID-19 (as evidenced by hypoxia and an inflammatory state) may undergo an optional second randomisation between either no additional treatment or tocilizumab[5]
.
On 11 May 2020, the trial was extended to paediatric patients with age restrictions for some arms:
- Hydroxychloroquine: infants with postnatal age of <180 days excluded
- Lopinavir-ritonavir: preterm infants with a corrected gestation age of <42 weeks or neonates with postnatal age of <14 days excluded[5]
.
Results from the dexamethasone arm of the trial were published on 16 June 2020, with researchers concluding that dexamethasone should now become standard of care in patients who require oxygen treatment.
The mechanisms for these medicines can be seen in Figure 1[8]
.
Sources and selection criteria
A search of national and international publications was undertaken using the Embase and Medline databases, the medicines listed in the WHO International Clinical Trials Registry Platform and the following search terms: coronavirus; severe acute respiratory syndrome coronavirus 2; 2019-nCoV; SARS-CoV-2; SARS-CoV; MERS-CoV and COVID-19. Articles were restricted to those published in English and although the initial literature search was undertaken in February 2020, subsequent publications after this date (from February – June 2020) have been identified and included. The authors independently reviewed the titles and abstracts for inclusion, including case reports that initially highlighted COVID-19 infection. Further relevant articles were identified from the citations referenced in articles reviewed. Active clinical trials were identified using: the disease search term ‘coronavirus infection’ on ClinicalTrials.gov; the index of studies of novel coronavirus pneumonia in the Chinese Clinical Trial Registry; and all COVID-19 registered trials listed on the NHS Health Research Authority website. Articles relating to MERS and SERS were also deemed necessary for inclusion.
Medicines currently being fast-tracked through clinical trials
There are no medicines currently approved to treat or prevent COVID-19, and no randomised controlled trial evidence that any treatment beyond best supportive care improves outcomes. However, many medicines are being investigated as part of nationally prioritised clinical trials.
The following sections of this rapid review provide an overview of these new and repurposed medicines, including their mechanism of action (see Figure 1[8]
), the rationale for potential treatment of COVID-19 and the current evidence base, as well as important practical considerations and implications for pharmacists involved in ongoing trials.
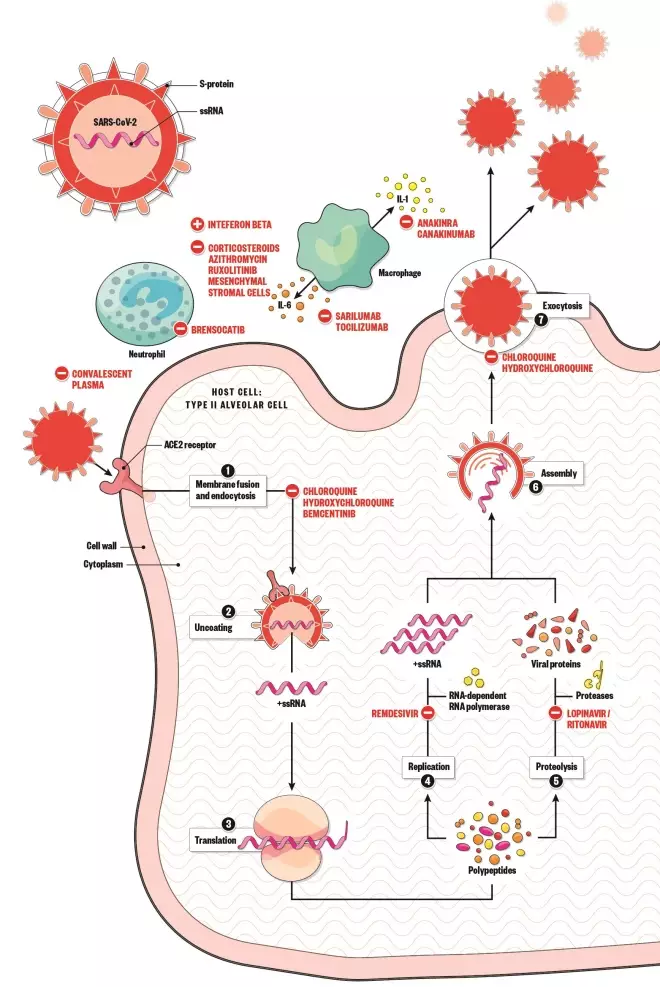
Figure 1: Mechanism of action for several medicines that are being put through clinical trial
Source: Alisdair MacDonald
1. Spike protein on the surface of SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry to the host cell 2. The virus releases its ribonucleic acid (RNA) 3. RNA is translated into polypeptides 4. Some polypeptides form RNA-dependent RNA polymerase, which is needed to make more RNA 5. Other polypeptides are cleaved by proteases to produce viral proteins 6. Proteins and RNA are assembled into a new virion 7. New virion is released from the host cell
Remdesivir
Mechanism of action
Remdesivir is an adenosine analogue that incorporates into nascent viral ribonucleic acid (RNA) chains and results in premature termination, preventing virus replication[3]
. It is widely distributed in the human body and is predominantly eliminated renally[9]
.
Rationale for use in the treatment of COVID-19
Remdesivir is under clinical development for the treatment of Ebola virus infection; however, it is not currently licensed globally for any indication[10]
. On 7 May 2020, remdesivir was approved for use in Japan and is now available in the United States under an emergency use authorisation[11],[12],[13]
. On 26 May 2020, the Medicines and Healthcare products Regulatory Agency (MHRA) gave the first positive scientific opinion under the Early Access to Medicines Scheme (EAMS) for use of remdesivir. Remdesivir will be available for patients in cases of high unmet medical need determined by a physician, where it will be provided to the NHS free of charge by Gilead throughout the EAMS period. It will also continue to be used in clinical trials[14]
. This does not replace the normal licensing procedures for medicines, but supports prescribers and patients to decide on whether to use the medicine before its licence is approved[11],[12],[13]
.
A recent review summarises the activity of remdesivir in vitro and in vivo activity in animal models against the coronaviruses that cause MERS and SARS, which are structurally similar to COVID-19[15]
. Two published in vitro studies have shown the potential effectiveness of remdesivir in treating COVID-19 and Ebola virus[3],[16]
.
Pre-clinical trials
Wang et al. studied the in vitro effects of varying concentrations of test drugs, including remdesivir, on COVID-19 infected Vero E6 cells[3]
. The study demonstrated that both remdesivir and chloroquine potently blocked virus infection at low-micromolar concentration and showed high selectivity index (i.e. a ratio that measures the window between cytotoxicity and antiviral activity)[17]
.
Clinical trials
A case report, authored by Holshue et al., of a man aged 35 years with COVID-19, who was treated with intravenous remdesivir on day 7 of hospitalisation (day 11 of infection) in the United States, was published on 5 March 2020[18]
. The authors do not comment on the dose used. By day eight, the patient’s clinical condition had improved and, after two weeks, the patient was reported to be afebrile, with all other symptoms resolved — except his cough, which was decreasing in severity[18]
.
In February 2020, Gilead initiated two phase III clinical trials to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19 in China[20],[21]
. The first was a phase III randomised, double-blind, placebo-controlled, multicentre study to evaluate the efficacy and safety of remdesivir in hospitalised patients with mild and moderate COVID-19 respiratory disease[20]
. This trial started on 12 February 2020, but was suspended in April 2020 because, at the time of writing, the epidemic in China was felt to be well controlled and, therefore, eligible patients could no longer be recruited[20]
.
The second, phase III, randomised, double-blind, placebo-controlled, multicentre study to evaluate the efficacy and safety of remdesivir in hospitalised patients with severe COVID-19 respiratory disease began on 6 February 2020[21]
. Only adults were included in the trial (at all sites). Patients received continued standard of care therapy (based on undefined criteria) together with remdesivir 200mg on day 1, followed by 100mg once-daily for 9 days, depending on which arm of the study the patient was randomised to[21]
. Despite this three-month trial initially having an estimated study completion date of early May 2020, the trial was also terminated in April 2020 as no eligible patients could be recruited.
Remdesivir is currently being used in the UK as part of both global and European clinical trials. The first is the ‘Adaptive COVID-19 Treatment Trial’ (ACTT), which has recruited over 1,000 patients globally and is being led in the UK and EU by the Medical Research Council (MRC) Clinical Trials Unit and University College London (UCL)[22],[23]
. The UCL-led part of the trial has recruited 79 patients, including 46 from the UK and was closed to new enrolments on 19 April 2020. High-level results for the first stage of the trial were reported on 29 April 2020 and showed that remdesivir decreased recovery time in patients with COVID-19[23]
. This resulted in a fast track emergency approval by the US Food and Drug Administration (FDA) of remdesivir for patients hospitalised with COVID-19[24]
.
The UK is also part of a multi-centre, adaptive, randomised trial of the safety and efficacy of treatments of COVID-19 in hospitalised patients (DisCoVeRy), which also has recruitment sites in France and Luxembourg[25]
. The trial compares use of remdesivir 200mg administered intravenously on day one, followed by 100mg once-daily thereafter for the duration of the hospitalisation, up to a ten-day total course, with either lopinavir-ritonavir; lopinavir-ritonavir with interferon ß-1a; hydroxychloroquine; and normal standard of care. The DisCoVeRy trial has an estimated study completion date of March 2023[25]
.
Safety and practical considerations
Clinical safety data from patients with Ebola virus disease treated with multiple-dose intravenous administration of remdesivir showed the drug was well tolerated[19]
. It can, however, increase liver enzymes between 5 and 25 days after initiating treatment[20],[21]
. In addition, reversible Grade 1 or 2 alanine aminotransferase or aspartate aminotransferase (AST) elevations were observed in several patients without abnormalities in total bilirubin, alkaline phosphatase, or albumin[21]
.
Remdesivir did not show any effects on renal function in the multiple-dose study on patients with Ebola virus disease[19]
. However, the formulation contains sulfobutylether-β-cyclodextrin, which is a solubilising agent, and should be used with caution in patients with renal impairment. Other reported adverse effects include infusion site phlebitis (i.e. inflammation of the vein) owing to the drug pH of 3–4, as well as headache, nausea, dyspepsia and constipation[10]
. Patients with severe liver disease (e.g. Child-Pugh score ≥ C, AST > five times the upper limit) and/or severe renal impairment (e.g. estimated glomerular filtration rate ≤30mL/min/1.73m2), or receiving continuous renal replacement therapy, haemodialysis or peritoneal dialysis are excluded from both of Gilead’s phase III trials[20],[21]
.
Based on the rapid distribution, metabolism and clearance of remdesivir, the likelihood of clinically significant interactions is low[9]
. However, remdesivir should not be co-administered with carbamazepine, phenobarbitone, phenytoin, primidone or rifampicin as these are enzyme inducers and can reduce blood plasma concentrations of the medicine[9]
. For a full list of drug interactions, pharmacists should consult the University of Liverpool COVID-19 Drug Interactions website[9]
.
Other considerations
Remdesivir has major patents across the world that last until 2038[26]
. Gilead has increased production of remdesivir to meet the demand for use in COVID-19 trials, but there is concern that many countries will simply not be able to afford this[14]
.
Favipiravir
Mechanism of action
Favipiravir, also known as T-705, is an antiviral drug developed in Japan in 2002 that inhibits viral RNA-dependent RNA polymerase, preventing the virus from replication[27]
.
Rationale for use in the treatment of COVID-19
Favipiravir is currently only licensed in Japan for influenza[28]
. As drug discovery takes a significant length of time, there is interest to examine whether existing antiviral medicines — particularly their mechanism of action — would be effective in treating coronaviruses, such as COVID-19.
Clinical trials and studies
An open-label, non-randomised, control study, conducted by Cai et al. in the isolation ward of the national clinical research centre for infectious diseases in Shenzhen, China, examined the effects of favipiravir versus lopinavir-ritonavir for the treatment of COVID-19[29]
. The dose of favipiravir used was 1600mg twice-daily on day 1 and 600mg twice-daily on days 2–14. The dose of lopinavir-ritonavir used was 400mg lopinavir and 100mg ritonavir twice-daily. Both favipiravir and lopinavir-ritonavir were continued until either viral clearance was confirmed or until 14 days had passed. A total of 35 patients were included in the favipiravir (experimental) arm and 45 patients in the lopinavir-ritonavir (control) arm. The authors reported that patients receiving favipiravir had a faster viral clearance and a higher improvement rate in chest imaging compared to those in the control arm. Interestingly, there were more adverse events observed in the control arm than in the experimental arm and no patients needed to discontinue favipiravir treatment[29]
.
Favipiravir is also being investigated in three active trials in China, with all three trials using the same dose of favipiravir for 7–14 days. The first is a clinical trial of favipiravir combined with chloroquine phosphate for the treatment of COVID-19 pneumonia[30]
. The trial commenced on 5 March 2020, has a proposed study completion date of 25 June 2020 and aims to recruit 150 participants. The study has three treatment arms: 1) favipiravir plus chloroquine phosphate (favipiravir dose 1600mg twice-daily on day 1 and 600mg twice-daily on days 2–10, with chloroquine phosphate 500mg twice-daily on day 1, 500mg once-daily on days 2–3 and 250mg once-daily on days 4–10); 2) favipiravir alone (dose as in treatment arm 1); and 3) placebo[30]
.
The second trial aims to evaluate the efficacy and safety of favipiravir combined with tocilizumab for the treatment of COVID-19[31]
. This trial is still actively recruiting. Participants are randomised to three treatment arms: 1) favipiravir with tocilizumab (favipiravir dose 1600mg twice-daily on day 1 and 600mg twice-daily on days 2–7, plus tocilizumab where the first dose is between 4 to 8mg/kg and the recommended dose is 400mg); 2) favipiravir alone (dose as in treatment arm 1); and 3) placebo[31]
.
Lastly, the third trial aims to evaluate the mechanism, clinical outcome, and therapeutic efficacy with favipiravir of COVID-19 patients whose nucleic acids changed from negative to positive[32]
. Participants are randomised either to favipiravir (dose 1600mg twice-daily on day 1 and 600mg twice daily on days 2–7; the maximum number of days taken is 14) or regular treatment (treatments other than lopinavir and ritonavir, chloroquine phosphate, hydroxychloroquine sulfate, umifenovir and colomycin can be given). The trial aims to recruit around 210 participants and has an estimated study completion date of 15 September 2020[32]
.
Safety and practical considerations
Transient thrombocytopenia (abnormally low levels of platelets), elevated liver enzyme levels and lipaemia (abnormally high blood concentration of emulsified fat) was found in an animal study of Lassa virus infection using an elevated dosing regimen of favipiravir[33]
.
Use of favipiravir in an Ebola virus patient, reported by Chinello et al. suggests that when administered at high doses, it may cause corrected QT (QTc) interval prolongation[34]
.
Favipiravir is known to be teratogenic; therefore, administration should be avoided in women if pregnancy is confirmed or suspected[28],[27]
.
Data on drug interactions is extremely limited; however, one in vitro study suggests that there may be an interaction between favipiravir and paracetamol[35]
. Although Zhao et al. concluded the interaction is unlikely to be clinically significant, they suggest that a conservative clinical recommendation would be to limit maximum daily paracetamol dosage to 3g (rather than the conventional 4g) in patients concomitantly taking favipiravir[35]
.
Favipiravir may potentially increase the exposure of cephalexin, flucloxacillin and penicillins[9]
. Concentrations of estradiol, ethinylestradiol and progesterone may also be affected by favipiravir[9]
.
Other considerations
There is also some data that suggests non-Asian people may need higher doses of favipiravir, but this is difficult to conclude as the phase I pharmacokinetic studies on favipiravir were conducted in Japan for influenza and in Guinea for Ebola[36]
.
Favipiravir is available as 200mg tablets and the WHO has reviewed the available evidence to consider whether favipiravir (either a loading dose of 1600mg or 1800mg followed by either 600mg three times per day or 800mg twice per day for 14 days) should be included in the Solidarity trial. At the time of the WHO’s consultation, which was published on 10 April 2020, it was felt that additional pre-clinical data were required. Favipiravir may have some benefits in combination with other antivirals to boost antiviral activity or decrease resistance; however, given the potential regimen, consideration needs to be given to the number of tablets a patient would have to take in a day and whether this is practical[36]
.
Chloroquine
Mechanism of action
Chloroquine is an aminoquinoline derivative with demonstrable anti-malarial and anti-inflammatory properties. The mechanisms of action of chloroquine across its diverse range of indications are disparate and are not fully understood. In vitro studies have shown that chloroquine and hydroxychloroquine can both inhibit SARS-CoV-2 transmission through alkalinisation of the intracellular phagolysosome, which prevents virion fusion and uncoating and, therefore, viral spread[3],[37],[38]
.
Clinical trials
On 13 May 2020, the ‘(Hydroxy)Chloroquine RepurpOsing to healthWorkers for Novel CORONAvirus mitigaTION (CROWN CORONATION) international, multi-site, Bayesian platform adaptive, randomised, double-blind, placebo-controlled trial’ assessing the effectiveness of varying doses of oral chloroquine was approved by the US National Institute for Health Research[39],[40]
. The trial is funded by the Bill & Melinda Gates Foundation to prioritise the protection of healthcare workers and aims to enlist 55,000 participants worldwide. Those participating will be randomised into one of four treatment arms: low-dose (300mg chloroquine base [i.e. the drug without the salt] weekly), medium-dose (300mg chloroquine base twice-weekly), high-dose (150mg chloroquine base daily) or placebo. In all treatment arms, an induction dose of 1200mg chloroquine base (or the equivalent number of placebo tablets in the placebo arm) will be taken in four divided daily doses (that is 300mg chloroquine base per day for four days) before starting the low-, medium-, or high-dose regimen. The trial is not yet recruiting at the time of publication of this review[40]
.
Rationale for use in the treatment of COVID-19
Based on their results from in vitro studies, Vincent et al. propose that both SARS and COVID-19 rely on interaction with angiotensin-converting enzyme 2 (ACE2) receptors for cell entry, and that chloroquine may interfere with terminal glycosylation of ACE2 receptors, effectively inhibiting virus entry[41]
. Indeed, several in vitro studies have investigated the potential of chloroquine for treating coronaviruses with a focus on SARS, MERS, and COVID-19 infections respectively; however, owing to the side effect profile and tolerability, most of the current clinical trials currently taking place choose to use hydroxychloroquine over chloroquine — these are described below[3],[42],[43]
.
Hydroxychloroquine sulphate
Mechanism of action
Hydroxychloroquine is thought to block virus infection by increasing endosomal pH required for cell fusion, therefore preventing virion fusion[44]
. In addition, it has been suggested that it may have immunomodulatory effects, but its full mechanism of action remains unclear[45]
.
Rationale for use in the treatment of COVID-19
Part of the rationale in considering the use of hydroxychloroquine came from clinical experience of using chloroquine with SARS and MERS [46]
. At present, in vitro studies show activity against SARS, COVID-19 and other coronaviruses, with hydroxychloroquine having higher potency against COVID-19 in comparison to chloroquine[37]
.
Clinical trials and studies
One small study of 36 patients, conducted by Gautret et al. in France reported that participants treated with hydroxychloroquine alone, or in combination with azithromycin, had reduced detection of COVID-19 RNA in upper respiratory tract specimens when compared with a non-randomised control group[47]
. However, in addition to the small sample size, there were differences in baseline viral load between the hydroxychloroquine group versus the combination treatment group. Patients were included in a single arm protocol between 1-16 March 2020 and received 200mg of hydroxychloroquine three times per day. The 20 participants treated with hydroxychloroquine showed significant reduction of viral carriage and more efficient viral elimination with azithromycin added[47]
. The results show hydroxychloroquine is efficient in clearing viral nasopharyngeal carriage of COVID-19 in most patients within three to six days. A statistically significant difference was noted between hydroxychloroquine/azithromycin-treated patients and controls starting from day 3 onwards (see Figure 2)[47]
.
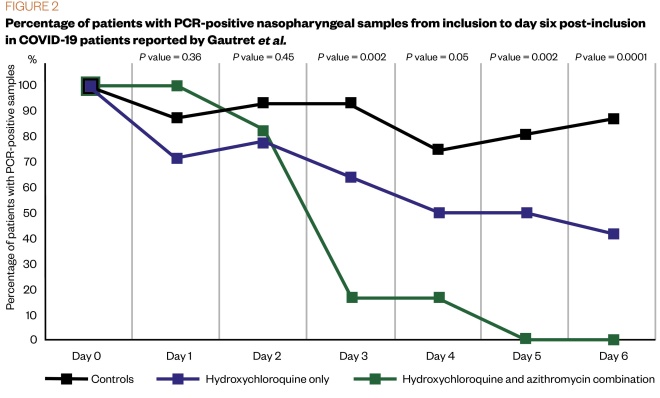
Figure 2: Percentage of patients with PCR-positive nasopharyngeal samples from inclusion to day 6 post-inclusion in COVID-19 patients reported by Gautret et al.
Source: International Journal of Antimicrobial Agents
[47]
PCR: polymerase chain reaction
A study conducted by Chen et al. in China aimed to assess the effectiveness of hydroxychloroquine in the treatment of patients with COVID-19[48]
. In this study, 15 patients received 400mg daily of hydroxychloroquine for 5 days, along with other conventional treatments (which were not specified in the trial documentation), while 15 patients received just conventional treatments. On day 7, 13 patients in the hydroxychloroquine group returned negative COVID-19 nucleic acid throat swabs, in comparison to the control group that received 14 negative swabs within the same period. Data were collected to determine the length of time it took for body temperature to return to normal or the number of participants with disease progression as per CT scans[48]
. No significant differences were observed across the two arms.
Between the control and hydroxychloroquine group, the average time it took for the participant’s body temperature to normalise was statistically comparable[48],[49]
. However, it is difficult to distinguish whether participants in these trials were solely on one regime of medication or a combination of drugs; therefore, it may be difficult to establish the efficacy of hydroxychloroquine from these results[48],[49]
.
Hydroxychloroquine was also being evaluated in the RECOVERY trial, but this was stopped as no clinical benefits were observed[6],[7]
. Participants randomised to this arm received an initial oral dose of 800mg, followed by 800mg 6 hours later, 400mg 6 hours later, 400mg 12 hours later and another 400mg given every 12 hours thereafter for a total of 10 days. All eligible participants had an electrocardiogram prior to treatment and those with a QTc interval of 450ms or over were excluded, along with those already on a macrolide antibiotic (e.g. azithromycin)[5]
. Hydroxychloroquine is also part of the DisCoVeRy trial.
The double-blind, randomised, placebo-controlled ‘Chloroquine/Hydroxychloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting (COPCOV)’ study, initially announced by the University of Oxford on 29 April 2020, has now been paused[50],[51]
. The study aimed to recruit 40,000 healthcare workers with confirmed or suspected COVID-19 from Europe, Africa and Asia. As part of the original trial protocol, participants could be followed up for a further five months and were randomised to either receive chloroquine/hydroxychloroquine or a placebo. The treatment group received a loading dose of 10mg base/kg (four 155mg tablets for a 60kg subject), followed by 155 mg daily (250mg chloroquine phosphate salt/200mg hydroxychloroquine sulphate) for 90 days. If participants were diagnosed with COVID-19, they would continue to take the study medication unless advised to stop by their healthcare professional, or for 90 days after enrolment, whichever is sooner. On 22 May 2020 the MHRA asked to pause recruitment to the COPCOV trial owing to ongoing concerns around hydroxychloroquine[50]
.
Cambridge University Hospitals NHS Foundation Trust also initiated a double-blind, randomised, placebo-controlled trial in a cohort of frontline healthcare workers, who may potentially be exposed to COVID-19[52],[53]
. Participants will be randomised to one of three arms and receive either: hydroxychloroquine daily (loading phase: 800mg for first 2 days; maintenance phase: a 200mg tablet once-daily) plus a weekly placebo, hydroxychloroquine weekly (loading phase: 800mg for first 2 days; maintenance phase: two 200mg tablets every seventh day/weekly ) plus a daily placebo; or placebo (daily and weekly)[3],[52],[53]
. Participants will be reviewed at an interim visit six weeks after baseline and again at the end of the study, around 90 days after randomisation. Participants will also complete brief questions about their health weekly while on treatment (i.e. remotely via app/web/phone). At the time of publication, this study has been temporarily halted.
Lastly, Sanofi are sponsoring a UK trial that aims to evaluate the effect of hydroxychloroquine with standard of care, compared with standard of care only on oxygen saturation/fraction of inspired oxygen (SpO2/FiO2) ratio in adult patients hospitalised with moderate to severe COVID-19[52],[53],[54]
. Information on doses and recruitment are not currently specified on the European Union Clinical Trials Register[53]
.
Safety and practical considerations
The most commonly reported side effects associated with hydroxychloroquine include nausea and abdominal pain, headaches and rashes. Rare side effects include bruising or infection[45]
. It is to be noted that a very rare side effect is toxicity to the back of the eyes, which is usually only seen when hydroxychloroquine has been taken for over five years[45]
. As with all immunosuppressant medication, recommendations on safe sun exposure should be followed, due to an increased risk of long-term skin damage[45]
.
The FDA released a drug safety communication on the 24 April 2020, stating that hydroxychloroquine and chloroquine can cause abnormal heart rhythms, such as QT interval prolongation and ventricular tachycardia[55]
. These risks may increase when these medicines are combined with other medicines known to prolong the QT interval, for example azithromycin, and the FDA state that these drugs should therefore only be used in a clinical trial setting for treating COVID-19[55]
.
A retracted observational study by Mehra et al., using a multinational registry across six continents, examined the effects of both hydroxychloroquine and chloroquine on COVID-19 patients who have been hospitalised[56]
. The authors reported that among patients receiving the drug, when used alone or with a macrolide, there was decreased in-hospital survival and an increased frequency of ventricular arrhythmias when used for treatment of COVID-19. As a result, the MHRA published concerns relating to the use of hydroxychloroquine as a treatment for patients with COVID-19[57]
.
On 23 May 2020, the executive group of the Solidarity trial implemented a temporary pause of the hydroxychloroquine arm within the trial, while the safety data was reviewed by the Data Safety Monitoring Board[4]
. The WHO has stated, however, that both hydroxychloroquine and chloroquine are accepted as generally safe for use in patients with autoimmune diseases or malaria[4]
. On 3 June 2020, the WHO announced that on the basis of the available mortality data, the members of the committee recommended that there are no reasons to modify the trial protocol. The executive group received this recommendation and endorsed the continuation of all arms of the Solidarity trial, including hydroxychloroquine[4]
.
Hydroxychloroquine is known to interact with several medications, including increasing plasma digoxin levels, enhancing the effects of hypoglycaemic treatments and impairing antiepileptic drug activity if given concurrently[45]
.
Caution should be used with concomitant use of QT prolonging agents. Please refer to the British National Formulary and the electronic medicines compendium for a complete list of interactions[45],[58]
.
Contraindications to hydroxychloroquine include hypersensitivity to the active substance; 4-aminoquinoline compounds; pre-existing maculopathy of the eye; Lapp lactase deficiency; glucose-galactose malabsorption; pregnancy; and children aged less than six years[45]
.
Other considerations
There are conflicting reports in the media about the use of both chloroquine and hydroxychloroquine for the treatment of COVID-19. The study by Mehra et al. prompted the exclusion of hydroxychloroquine from the Solidarity trial[56]
. However, the authors stated that there are several limitations to their findings, such as the presence of cardiovascular comorbidity in the study population, which could partially explain the observed risk of increased cardiovascular toxicity with the use of chloroquine or hydroxychloroquine, especially when used in combination with macrolides. There is the possibility of unmeasured confounding factors and uncertainty over whether the association of increased risk of in-hospital death with use of the drug regimens is linked directly to cardiovascular risks. In addition, the authors did not conduct a drug dose-response analysis of the observed risks and the findings should therefore be interpreted with caution[56]
. On 6 June 2020, three of the study’s four authors made the decision to retract this study after they were unable to independently verify the data used for their analysis[56]
. The implications of this will add to the controversy surrounding hydroxychloroquine.
Lopinavir-ritonavir
Mechanism of action
Lopinavir and ritonavir inhibit protease, an enzyme that HIV and coronaviruses use to replicate. In HIV, inhibition of HIV protease prevents cleavage of the gag-pol polyprotein, resulting in the production of immature, non-infectious virus[16],[59]
. Ribavirin, a synthetic nucleoside analogue, has been used in combination with lopinavir and ritonavir and is said to work against viruses[60]
.
Rationale for use in the treatment of COVID-19
Lopinavir showed in vitro antiviral activity against SARS; however, when combined with ribavirin, the concentration of lopinavir increased, suggesting ribavirin works synergistically[60]
.
Lopinavir–ritonavir in combination with ribavirin also showed reduced fatality rate and milder disease course during an open clinical trial in 152 patients admitted to the United Christian Hospital and Caritas Medical Centre, Hong Kong, during the 2003 SARS outbreak[60]
.
Clinical trials and studies
A randomised, controlled, open-label trial involving 199 hospitalised adult patients with confirmed COVID-19 infection conducted by Cao et al. randomised patients into two arms; 99 were assigned to the lopinavir-ritonavir group, and 100 to standard care[61]
. The primary endpoint was the time to clinical improvement, defined as the time from randomisation to either an improvement of two points on a seven-category ordinal scale or discharge from the hospital, whichever came first. The exact descriptions of standard care were not provided as this varied between patients, but generally consisted of supplemental oxygen, non-invasive and invasive ventilation, antibiotic agents and extracorporeal membrane oxygenation. Results obtained from patients taking lopinavir-ritonavir showed no difference from standard care in the time to clinical improvement, with the mortality at 28 days somewhat similar in both groups (19.2% with lopinavir-ritonavir and 25.0% in the standard care group)[61]
. In those who were hospitalised with severe COVID-19, no benefit was observed in treatment with lopinavir–ritonavir beyond standard care.
A cohort study by Young et al. used lopinavir–ritonavir at different doses to understand the clinical course in 18 COVID-19 patients in Singapore[62]
. Of the 18 patients, 5 were treated with lopinavir-ritonavir (200mg/100mg) twice-daily, which is half the usual dose of lopinavir. Of these patients, the clinical condition of two deteriorated. The low dose of lopinavir-ritonavir used in this study and the limited number of participants may explain the results; however, only when additional larger randomised trials are undertaken will any possibility of benefits of treatment be established.
Lopinavir-ritonavir has also been included as a treatment arm within the RECOVERY trial[5]
. Lopinavir 400mg with ritonavir 100mg will be given orally every 12 hours for 10 days. To avoid depleting the current available stock for patients using the drugs for conditions such as HIV, the trial supply will be sourced from Public Health England[5]
.
Safety and practical considerations
Information surrounding the synergistic abilities of ribavirin and lopinavir-ritonavir is limited. This may be owing to a lack of comprehensive understanding of the mechanism of action of this combined treatment and reflects the limited studies undertaken at present.
Side effects of lopinavir-ritonavir include — but are not limited to — anaemia, angioedema, diarrhoea, dizziness, dry mouth, increased risk of infection, menstrual cycle irregularities, myopathy, Stevens-Johnson syndrome, QT prolongation and torsade de pointes, atrioventricular block, PR prolongation, renal failure and hepatotoxicity[61],[59],[63]
. In light of the number of interactions that can occur with concomitant use, pharmacists should refer to https://www.covid19-druginteractions.org/ for further information.
Other considerations
Data on the use of ribavirin with lopinavir-ritonavir is very limited, with results from only in vitro studies currently published. Although Chu et al. used ribavirin alone to treat coronaviruses, it is not being used in any current COVID-19 trial. The efficacy of triple therapy for the treatment of SARS and COVID-19 is yet to be fully studied and documented in humans[60]
.
Corticosteroids
A class of steroid hormones, corticosteroids are a range of chemically related compounds that can be classified according to their glucocorticoid or mineralocorticoid effects. They are among the most potent anti-inflammatory and immunosuppressive drugs available to treat a range of indications[64]
.
Mechanism of action
Corticosteroids have a good inhibitory effect on inflammatory factors. The main anti-inflammatory effect of glucocorticoids is to inhibit many pro-inflammatory genes that encode cytokines, chemokines, cell adhesion molecules, inflammatory enzymes, and receptors to address the inflammatory process and restore homeostasis[65]
.
Corticosteroids, particularly glucocorticoids, used in the treatment of inflammation generally target or influence the actions of inflammatory proteins. They increase the rate of synthesis of anti-inflammatory proteins while attenuating pro-inflammatory protein synthesis.
Rationale for use in the treatment of COVID-19
Owing to their potential effect on the rate of synthesis of both anti-inflammatory and pro-inflammatory proteins, in theory, corticosteroids may have a role in limiting or suppressing lung inflammation in COVID-19[66]
.
Patients infected with MERS or SARS have previously been treated with high-dose corticosteroids; however, reported outcomes in key studies demonstrated greater harm than benefit[67],[68],[69],[70],[71]
. Documented complications associated with this strategy include delayed clearance of viral RNA from the respiratory tract, psychosis, avascular necrosis and diabetes[67],[68],[69],[70],[71]
. However, evidence from a 2006 retrospective cohort study of 401 SARS-infected patients by Chen et al. found that low-to-moderate doses of steroids reduced mortality and duration of hospital stay[72]
.
A more recent systematic review and meta-analysis by Yang et al. reaffirmed the need for caution when using these drugs to treat COVID-19[73]
. Results from 5,270 patients from 15 studies indicated that corticosteroids are generally associated with higher mortality, though corticosteroids are more likely to be used as treatment for patients with severe illness[73]
. The authors concluded that corticosteroids are not recommended for patients with mild manifestations of COVID-19 as, with increasing severity of infection, the greater the potential benefit of their anti-inflammatory actions[73]
. Treatment of COVID-19 with corticosteroids and the associated immune suppression has been shown to cause higher rates of bacterial infection and other complications, thus consolidating the need for caution[73]
. The main limitation of this meta-analysis was a lack of high-level evidence as the included studies did not involve randomised controlled trials[73]
.
Clinical trials
Several corticosteroids including methylprednisolone, dexamethasone, prednisolone and hydrocortisone have been used in clinical trials. A multi-centre, prospective, randomised, and open-label study in China aims to evaluate whether methylprednisolone improves the prognosis of patients with COVID-19 infection and will compare the degree of lung injury (as quantified by a Murray Lung Injury Score) between patients receiving 40mg methylprednisolone every 12 hours for five days versus patients receiving standard, supportive therapy[74]
. Results of this study are not yet available.
The RECOVERY trial includes low-dose dexamethasone in one of the five treatment arms[5]
. Patients randomised for this treatment receive 6mg of steroid expressed as dexamethasone base, in either intravenous or oral form. However, pregnant women instead receive either 40mg oral prednisolone once-daily or 80mg intravenous hydrocortisone twice-daily. Eligible patients are being treated for up to ten days or until discharge, whichever is shorter. The primary outcome for this study is all-cause mortality at 28 days after randomisation[5]
. Recruitment of adult patients to the dexamethasone arm of the RECOVERY trial closed on 8 June 2020 owing to sufficient adult enrolment levels. However, at present, children will still be recruited into this arm[75]
.
Results from this arm of the RECOVERY trial were published on 16 June 2020. A total of 2,104 patients were randomised to receive dexamethasone 6mg once-daily (either by mouth or by intravenous injection) for 10 days and were compared with 4,321 patients randomised to usual care alone. Among the patients who received usual care alone, 28-day mortality was highest in those who required ventilation (41%), intermediate in those patients who required oxygen only (25%), and lowest among those who did not require any respiratory intervention (13%).
The results show that dexamethasone reduced deaths by a third in ventilated patients (rate ratio 0.65 [95% confidence interval 0.48 to 0.88]; P =0.0003) and by a fifth in other patients receiving oxygen only (0.80 [0.67 to 0.96]; P =0.0021). However, there was no benefit among those patients who did not require respiratory support (1.22 [0.86 to 1.75]; P =0.14).
Safety and practical considerations
Knowledge of the glucocorticoid and mineralocorticoid activity of individual steroids is important when screening these drugs in the clinical setting. These properties can influence a variety of clinical factors including fluid and electrolyte balance (e.g. dexamethasone is a high-potency glucocorticoid with negligible mineralocorticoid effects)[76]
. Methylprednisolone and prednisolone have predominantly glucocorticoid effect, while hydrocortisone has relatively higher mineralocorticoid activity[64]
.
Steroids differ in their anti-inflammatory potencies, kinetic and biological half-lives[76]
. They may also inhibit immune responses as discussed above. Where rapid and intensive glucocorticoid effect is required, intravenous over oral treatment may be indicated. As intravenous and oral dexamethasone is being used in the RECOVERY trial, consideration should be given to the pregnancy status of the patient as dexamethasone readily crosses the placental barrier[76]
. Therefore, either prednisolone or hydrocortisone should be used in pregnant patients as an alternative treatment. This is because both prednisolone and hydrocortisone are converted by the placenta into inactive forms (e.g. prednisolone is converted into inactive prednisone or cortisone)[5],[77]
.
Other considerations
Patients with coronaviruses treated with corticosteroids have experienced side effects owing to their immunosuppressive effects, meaning that caution must be exercised when considering using the treatment. It is also difficult to conclude whether the increased mortality observed in severely ill patients treated with corticosteroids is a function of the drugs used or the severity of illness[67],[73]
.
Interferons
A group of naturally occurring proteins, interferons are an important category of protein-based therapeutics — or biologics. They are a heterogeneous group of cytokines with complex biological functions and distinct characteristics, and broadly include type I interferons (interferon-α and interferon-β) and type II interferons (interferon-γ)[78]
.
Rationale for use in treatment of COVID-19
Endogenous interferons are produced in response to viral infections and modulate the immune response by exerting anti-viral activity. In addition, other coronaviruses — namely MERS and SARS — have previously been shown to be highly susceptible to interferon treatment in vitro
[79]
.
Interferon-α-2b, in PEGylated form (conjugated with polyethylene glycol [PEG]), has previously been investigated in a MERS retrospective cohort study examining the effects of ribavirin and interferon-based therapy (PEG-Interferon-α-2a, PEG-Interferon-α-2b and interferon-β-1a) on 90-day mortality and viral RNA clearance[80]
. Logistic regression and Cox-proportional hazard analyses (with respect to their corresponding odds ratio and hazard ratio outputs) indicated statistically significant increases in 90-day mortality in patients who received ribavirin/interferon treatment over those who did not receive these treatments. MERS RNA clearance was not significantly increased by the presence of either treatment[80]
.
In another MERS retrospective cohort study, multi-variate adjusted logistic regression results indicated a non-statistically significant difference in the odds of mortality when either interferon-α or interferon-β were administered[81]
.
Interferon-β1b has been investigated in both in vitro and in vivo non-human primate studies[82]
. In the latter, administration of interferon-β1b resulted in statistically significant reductions in mean viral (MERS) load and disease severity in treated versus non-treated primates[82]
.
Interferon-γ differs from interferon-β and interferon-α in that it is a type II interferon. It has been investigated in in vitro studies in combination with interferon-β, which illustrated synergistic and greater activity compared with use of either agent alone to reduce SARS replication in cell culture[83]
.
Clinical trials
At the time of publication, 2 UK-based trials could also be identified on the EU Clinical Trials Register and a total of 17 trials were documented on the American Clinical Trial Registry as investigating interferons for coronaviruses[84],[85]
. In the UK, a nebulised form of interferon-β formulated by Synairgen Research Limited is currently being used as part of an ongoing, randomised, double-blind and placebo-controlled trial to examine its efficacy in reducing the severity of lung infection associated with COVID-19[86]
. Patients receive either drug or placebo via inhalation once-daily for 14 days.
The ‘Randomized, Embedded, Multifactorial Adaptive Platform trial for Community-Acquired Pneumonia’ has 198 active sites with 1008 total patients randomised to date over 14 countries, including the UK[87]
. It now includes a new domain-specific for immune-modulating treatment, including interferon-β-1a, as part of the COVID-19 response. This has been included as many patients with COVID-19 infection present with community-acquired pneumonia. The drug will be administered intravenously through either a central or peripheral line once each day for six days or up to hospital discharge — whichever is shorter[87]
.
Safety considerations
Licensed medicinal interferons in the UK may broadly fall into one of the following categories: interferon-β; interferon-α; and interferon-γ1b. Interferon-β and interferon-α have also been produced in PEGylated forms to extend therapeutic half-life, and reduce clearance and immunogenicity[88]
.
The use of interferons is restricted to specialist settings. While their medicinal forms are expressed in terms of international units (IU)/mL, different preparations of interferon have different specific biological activities. For this reason, it is inappropriate to compare their antiviral actions using IU/mL[89]
.
Other considerations
Owing to their different specific biological activities, particular expertise is required to compare the varying antiviral actions of interferons. They are generally expensive to procure and interferon-β is currently only recommended by NICE for the treatment of its licensed indications on the condition that manufacturers provide them on discounted patient access schemes[90]
.
Discussion and conclusions
COVID-19 is a global pandemic that has caused more than 468,308 deaths worldwide[91]
. Researchers are currently investigating the potential role of more than 60 medicines as treatments for the disease. Although this article summarises the current evidence related to several of the main investigational drugs being used in clinical trials for the treatment of COVID-19, as of 23 June 2020 there are no licensed medications for the treatment of the disease.
The RECOVERY trial is of high importance to UK; however, it should be noted that:
- Not all trial arms may be appropriate for a patient (e.g. owing to contraindications based on comorbidities or concomitant medication);
- Within some trusts, not all treatment arms may be undertaken (e.g. owing to manufacturing and supply shortages);
- Not all treatment arms will always be active (e.g. owing to lack of relevant approvals and contractual agreements)[5]
.
This review has provided an overview of the main medicines currently being investigated in clinical trials for the potential treatment of COVID-19 and has highlighted the potential benefits and issues of their use in practice.
Some evidence indicates that remdesivir could be a promising treatment option for COVID-19, but data is limited and further study data is warranted. It is unknown how much remdesivir may cost if the therapy is used more widely for treatment , but Gilead are taking steps to expand its supply globally.
Although favipiravir is not currently included in any of the UK trials for COVID-19, it has the potential to be advantageous as it is a broad-spectrum antiviral, has antiviral activity against different RNA viruses and a high barrier for resistance. However, safety and potency issues need to be overcome before this drug could be used to treat large patient groups. The WHO continues to review and consider its inclusion in the Solidarity trial, and will share further information when available[36]
.
There is interest in the prospect of hydroxychloroquine in treating COVID-19, given the clinical experience of using chloroquine to treat other coronaviruses (e.g. SARS and MERS); however, current data is insufficient to draw any conclusions on the efficacy of hydroxychloroquine in the treatment of COVID-19. Owing to its removal from the RECOVERY trial, it is less likely that pharmacists in the UK will encounter this medicine.
There is limited information on the use of triple therapy of lopinavir-ritonavir combined with ribavirin in treating COVID-19. Until further research is carried out, evidence of the significance of the combination of these drugs remains inconclusive[5]
. In addition, until recently there was difficulty in acquiring liquid preparations, which would have prevented certain patient types from receiving treatment in all arms of trials that include this treatment. With this issue now resolved, there is an opportunity to increase the number of potential patients treated with lopinavir-ritonavir.
Furthermore, provisions should be put in place locally at a site level to prevent lopinavir-ritonavir and hydroxychloroquine being prescribed for patients who have not consented to inclusion in the RECOVERY trial. This presents an opportunity for pharmacists to work as part of multidisciplinary teams to ensure safe prescribing practices and optimum patient care.
High-level evidence in the form of randomised clinical trials is lacking and published research focusing on the clinical use of corticosteroids for treating coronaviruses is currently limited to observational or cohort studies. Corticosteroids exhibit both anti-inflammatory and immunosuppressant effects, which confers both benefits and risks in the context of treating COVID-19. The preliminary results of the RECOVERY trial show dexamethasone to be a low-cost, effective treatment for patients needing oxygen treatment. The results of other clinical trials will establish further evidence for this group of drugs.
Interferons are complex both endogenously and in pharmaceutical form; clinically they must be carefully used by those with experience in their use. Should interferon-β prove clinically useful in the treatment of COVID-19, there will be challenges in making it widely available owing to its high cost.
It is likely that overcoming COVID-19 will include a combination of measures, including these medicinal therapies currently under investigation. In addition, preventative therapeutic strategies, such as vaccines, may be in place to help curb the spread of the infection. Pharmacists’ contribution to managing the treatment of patients with confirmed or suspected COVID-19 is vital, given the potential drug interactions, side effects and contraindications.
In the current climate, patients admitted to hospital with COVID-19 infection can present with a wide range of clinical issues. Despite the lack of an effective COVID treatment, healthcare professionals can take actions to manage these associated issues safely and effectively. Considerations, such as the management of patients who present with swallowing difficulties or those who are intubated, will present issues related to the administration of medication and will require a pharmacist’s input and advice, as well as assistance with the administration of drugs via enteral feeding tubes[5]
. The role of pharmacists in antimicrobial stewardship will also be extremely important over the coming months, given that the use of antibiotics in treating pneumonia caused by COVID-19 will require diligent monitoring[92]
.
Investigational medicines are being used off-label or unlicensed as part of clinical trials in response to the pandemic; therefore, long-term data are required to evaluate their safety and efficacy. Doses used may also be higher than normal licensed doses, as with hydroxychloroquine. It is essential that pharmacists report any serious or unusual side effects observed by patients who are included in these trials and receiving any of these medicines.
Following a basic literature search strategy, this review does not intend to be a comprehensive collation of all relevant literature, but rather an overview of several relevant publications in this area of practice. It should be noted that the accuracy and coverage of this review will be short-lived owing to the rapid advances being made in this area; however, this rapid review has focused on the medicines that UK pharmacists are most likely to encounter in practice. Other medicines are currently being investigated, but these are outside the scope of this review.
Although clinical trials are ongoing, steps taken by the MHRA mean that remdesivir may be used on a wider basis in patients with COVID-19[93]
. During the current pandemic, there is the possibility that therapies may be fast-tracked through clinical trials and approved for treatment to provide the best care for patients.
About the authors
Ashifa Trivedi, lead divisional pharmacist women & children; Sadhna Sharma, senior rotational clinical pharmacist; Blend Ashtey, rotational clinical pharmacist; all within the Pharmacy Department at The Hillingdon Hospitals NHS Foundation Trust
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was used in the production of this manuscript.
Acknowledgment
The authors dedicate this work to the memory of NHS healthcare staff who have given their lives in the care of patients with COVID-19.
References
[1] World Health Organization. Updated WHO advice for international traffic in relation to the outbreak of the novel coronavirus 2019-nCoV. 2020. Available at: https://www.who.int/ith/2020-24-01-outbreak-of-pneumonia-caused-by-new-coronavirus/en/ (accessed June 2020)
[2] Zumla A, Chan JF, Azhar EI et al. Coronaviruses — drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37
[3] Wang M, Cao R, Zhang L, Yang X et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0
[4] World Health Organization. “Solidarity” clinical trial for COVID-19 treatments. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (accessed June 2020)
[5] University of Oxford. Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial. 2020. Available at: https://www.recoverytrial.net (accessed June 2020)
[6] Robinson J. RECOVERY trial to stop enrolling patients to ‘ineffective’ hydroxychloroquine arm. Pharm J. 2020. Available at: https://www.pharmaceutical-journal.com/news-and-analysis/news/recovery-trial-to-stop-enrolling-patients-to-ineffective-hydroxychloroquine-arm/20208044.article?firstPass=false (accessed June 2020)
[7] University of Oxford. Recovery. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. 2020. Available at: https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19 (accessed June 2020)
[8] Connelly D. Targeting COVID-19: the drugs being fast-tracked through clinical trials and how they work. Pharm J 304;(7937):312-313. doi: 10.1211/PJ.2020.20207949
[9] University of Liverpool. Interactions with experimental COVID-19 therapies. 2020. Available at: https://www.covid19-druginteractions.org/ (accessed June 2020)
[10] Wang Y, Zhang D, Du G et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020; 395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9
[11] Gilead. Gilead Announces Approval of Veklury (remdesivir) in Japan for Patients With Severe COVID-19. 2020. Available at: https://www.gilead.com/news-and-press/press-room/press-releases/2020/5/gilead-announces-approval-of-veklury-remdesivir-in-japan-for-patients-with-severe-covid19 (accessed June 2020)
[12] MHRA. Early access to medicines scheme (EAMS) scientific opinion: Remdesivir in the treatment of patients hospitalised with suspected or laboratory-confirmed SARS-CoV-2 infection who meet the clinical criteria. 2020. Available at: https://www.gov.uk/government/publications/early-access-to-medicines-scheme-eams-scientific-opinion-remdesivir-in-the-treatment-of-patients-hospitalised-with-suspected-or-laboratory-confirme (accessed June 2020)
[13] US Food and Drug Administration. Remdesivir EUA Letter of Authorization. 2020. Available at: https://www.fda.gov/media/137564/download (accessed June 2020)
[14] Gilead. Working to supply remdesivir for COVID-19. 2020. Available at: https://www.gilead.com/purpose/advancing-global-health/covid-19/working-to-supply-remdesivir-for-covid-19 (accessed June 2020)
[15] Amirian SE & Levy JK. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health 2020;9:100128. doi: 10.1016/j.onehlt.2020.100128
[16] Warren TK, Jordan R & Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016;531(7594):381–385. doi: 10.1038/nature17180
[17] Pritchetta JC, Naesens L & Montoya J. Treating HHV-6 infections: the laboratory efficacy and clinical use of anti-HHV-6 agents. In: Flamand L, Lautenschlager I, Krueger G, Ablashi D (eds.) Human Herpesviruses HHV-6A, HHV-6B & HHV-7 (Third Edition). Diagnosis and Clinical Management. Oxford: Elsevier; 2014. pp.311–331.
[18] Holshue ML, DeBolt C, Lindquist S et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020; 382:929-936. doi: 10.1056/NEJMoa2001191
[19] World Health Organization. WHO R&D Blueprint – Ad-hoc expert consultation on clinical trials for Ebola therapeutics. Deliberations on design options for randomized controlled clinical trials to assess the safety and efficacy of investigational therapeutics for the treatment of patients with Ebola virus disease. Appendix 4: Summaries of evidence from selected experimental therapeutics, as of October 2018. 2018. Available at: https://www.who.int/ebola/drc-2018/summaries-of-evidence-experimental-therapeutics.pdf
[20] US National Library of Medicine. A trial of remdesivir in adults with mild and moderate COVID-19. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04252664 (accessed June 2020)
[21] US National Library of Medicine. A trial of remdesivir in adults with severe COVID-19. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04257656 (accessed June 2020)
[22] US National Library of Medicine. Adaptive COVID-19 Treatment Trial. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04280705 (accessed June 2020)
[23] University College London. Preliminary results of COVID-19 drug treatment trial found to improve recovery. 2020. Available at: https://www.ucl.ac.uk/news/2020/apr/preliminary-results-covid-19-drug-treatment-trial-found-improve-recovery (accessed June 2020)
[24] US Food and Drug Administration. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. 2020. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment (accessed June 2020)
[25] US National Library of Medicine. Trial of treatments for COVID-19 in hospitalized adults (DisCoVeRy). 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04315948 (accessed June 2020)
[26] Prabhala A & Hoen E. We’ll find a treatment for coronavirus – but drug companies will decide who gets it. 15 April 2020. Available at: https://www.theguardian.com/commentisfree/2020/apr/15/coronavirus-treatment-drug-companies (accessed June 2020)
[27] Furuta Y, Komeno T & Nakamura T. Favipiravir (T-705), a broad-spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 2017;93(7):449–463. doi:10.2183/pjab.93.027
[28] Delang L, Abdelnabi R & Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003
[29] Cai Q, Yang M, Liu D et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020. Available at: https://doi.org/10.1016/j.eng.2020.03.007
[30] US National Library of Medicine. Clinical trial of favipiravir tablets combine with chloroquine phosphate in the treatment of novel coronavirus pneumonia. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04319900?term=favipiravir&cond=Coronavirus&draw=2&rank=1 (accessed June 2020)
[31] US National Library of Medicine. Favipiravir combined with tocilizumab in the treatment of corona virus disease 2019. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04310228?term=favipiravir&cond=Coronavirus&draw=2&rank=3 (accessed June 2020)
[32] US National Library of Medicine. Corona virus disease 2019 patients whose nucleic acids changed from negative to positive. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04333589?term=favipiravir&cond=Coronavirus&draw=2&rank=5 (accessed June 2020)
[33] Rosenke K, Feldmann H, Westover JB, et al. Use of favipiravir to treat Lassa virus infection in macaques. Emerg Infect Dis 2018;24(9):1696–1699. doi: 10.3201/eid2409.180233
[34] Chinello P, Petrosillo N, Pittalis S et al. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis. 2017;11(12):e0006034. doi: 10.1371/journal.pntd.0006034
[35] Zhao Y, Harmatz J, Epstein, C et al. Favipiravir inhibits acetaminophen sulfate formation but minimally affects systemic pharmacokinetics of acetaminophen. Br J Clin Pharmacol 2015;80(5):1076–1085. doi: 10.1111/bcp.12644
[36] World Health Organization. WHO R&D Blueprint. COVID-19 Informal consultation on the potential inclusion of Favipiravir in a clinical trial. 2020. Available at: http://origin.who.int/blueprint/priority-diseases/key-action/RDBlueprintbtxexpertgrouponFavipiravircallApril10th2020.pdf (accessed June 2020)
[37] Yao X, Ye F, Zhang M et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020. Available at: https://doi.org/10.1093/cid/ciaa237
[38] Liu J, Cao R, Xu M et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6:16. doi: 10.1038/s41421-020-0156-0
[39] National Institute for Health Research. Crown Coronation. (Hydroxy)Chloroquine RepurpOsing to healthWorkers for Novel CORONAvirus mitigaTION: An international, multi-site, Bayesian platform adaptive, randomised, double-blind, placebo-controlled trial assessing the effectiveness of varied doses of oral Chloroquin. 2020. Available at: https://www.nihr.ac.uk/covid-studies/study-detail.htm?entryId=282280 (accessed June 2020)
[40] US National Library of Medicine. CROWN CORONATION: Chloroquine RepurpOsing to healthWorkers for Novel CORONAvirus mitigaTION (CROWN CORONA). 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04333732 (accessed June 2020)
[41] Vincent MJ, Bergeron E, Benjannet S et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005;2:69. doi: 10.1186/1743-422X-2-69
[42] Keyaerts E, Vijgen L, Maes P et al. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085
[43] De Wilde AH, Jochmans D, Posthuma CC et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 2014; 58(8):4875–4884. doi: 10.1128/AAC.03011-14
[44] Owens B. Excitement around hydroxychloroquine for treating COVID-19 causes challenges for rheumatology. Lancet Rheumatol 2020;2(5):e257. doi: 10.1016/S2665-9913(20)30089-8
[45] Electronic medicines compendium. Plaquenil-Hydroxychloroquine sulfate 200mg film-coated tablets - Summary of Product Characteristics. 2020. Available at: https://www.medicines.org.uk/emc/product/1764/smpc (accessed June 2020)
[46] Dyall J, Coleman CM, Hart BJ et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 2014;58(8):4885–4893. doi:10.1128/AAC.03036-14
[47] Gautret P, Lagier J, Parol Pet al. Hydroxychloroquine and azithromycin as a treatment of COVIDâ€19: results of an openâ€label nonâ€randomized clinical trial. International Journal of Antimicrobial Agents online. 2020. doi:10.1016/j.ijantimicag.2020.105949
[48] Chen J, Liu D, Liu L et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. J Zhejiang Univ (Med Sci) 2020;49(2):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03
[49] Zhu N, Zhang D, Wang W et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017
[50] Mohidol Oxford Tropical Medicine Research Unit. COPCOV key messages. 2020. Available from https://www.tropmedres.ac/covid-19/copcov/copcov-key-messages (accessed June 2020)
[51] US National Library of Medicine. Chloroquine/hydroxychloroquine prevention of coronavirus disease (COVID-19) in the healthcare setting (COPCOV). 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04303507?term=copcov&draw=2 (accessed June 2020)
[52] NHS Health Research Authority. An adaptive phase 2/3, randomized, open-label study assessing efficacy and safety of hydroxychloroquine for hospitalized patients with moderate to severe COVID-19. 2020. Available at: https://www.hra.nhs.uk/covid-19-research/approved-covid-19-research/282362/ (accessed June 2020)
[53] EU Clinical Trials Register. Clinical Trials. 2020. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001270-29/GB (accessed June 2020)
[54] Cambridge University Hospitals NHS Foundation Trust. ChemoPROphyLaxIs For covId-19 infeCtious disease (the PROLIFIC trial). 2020. Available at: https://cambridgebrc.nihr.ac.uk/wp-content/uploads/2020/05/PROLIFIC_Protocol_V1.0-07April2020.pdf (accessed June 2020)
[55] US Food and Drug Administration. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. 2020. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or (accessed June 2020)
[56] Mehra M, Desai S, Ruschitzka G, Patel A. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet online. 2020. Available at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/fulltext (accessed June 2020)
[57] Medicines and Healthcare products Regulatory Agency. Chloroquine and hydroxychloroquine not licensed for coronavirus (COVID-19) treatment. 2020. Available at: https://www.gov.uk/government/news/chloroquine-and-hydroxychloroquine-not-licensed-for-coronavirus-covid-19-treatment (accessed June 2020)
[58] British National Formulary. Hydroxychloroquine sulfate. 2020. Available at: https://bnf.nice.org.uk/drug/hydroxychloroquine-sulfate.html (accessed June 2020)
[59] Electronics Medicines Compendium. Kaletra 200mg/50mg film-coated tablets — summary of product characteristics. 2020. Available at: https://www.medicines.org.uk/emc/product/221/smpc (accessed June 2020)
[60] Chu C, Cheng V, Hung I et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004;59:252-256. doi: 10.1136/thorax.2003.012658
[61] Cao B, Wang Y, Wen Det al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. New Engl J Med 2020;382(19):1787–1799.doi: 10.1056/NEJMoa2001282
[62] Young BE, Ong SWX, Kalimuddin S et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020;323(15):1488–1494. doi:10.1001/jama.2020.3204
[63] British National Formulary. Lopinavir with ritonavir. 2017. Available at: https://bnf.nice.org.uk/drug/lopinavir-with-ritonavir.html#nationalFunding (accessed June 2020)
[64] British National Formulary. Corticosteroids, general use. 2017. Available at: https://bnf.nice.org.uk/treatment-summary/corticosteroids-general-use.html (accessed June 2020)
[65] Cruz-Topete D & Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015;22(1–2):20–32. doi: 10.1159/000362724
[66] Russell C, Millar J & Baillie J. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473-475. doi: 10.1016/S0140-6736(20)30317-2
[67] Arabi YM, Mandourah Y, Al-Hameed F et al. Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC
[68] Lee N, Allen Chan KC, Hui DS et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006
[69] Lee DTS, Wing YK, Leung HCM et al. Factors associated with psychosis among patients with severe acute respiratory syndrome: a case-control study. Clin Infect Dis 2004;39(8):1247–1249. doi: 10.1086/424016
[70] Li YM, Wang SX, Gao HS et al. Factors of avascular necrosis of femoral head and osteoporosis in SARS patients’ convalescence. Zhonghua Yi Xue Za Zhi 2004; 84(16):1348–1353. PMID: 15387943
[71] Xiao JZ, Ma L, Gao J et al. Glucocorticoid-induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy. Zhonghua Nei Ke Za Zhi 2004;43(3):179–182. Chinese. PMID: 15059370.
[72] Chen RC, Tang XP, Tan SY et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441
[73] Yang Z, Liu J, Zhou Y et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect 2020;e13–e20. doi: 10.1016/j.jinf.2020.03.06
[74] US National Library of Medicine. Glucocorticoid therapy for COVID-19 critically ill patients with severe acute respiratory failure. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04244591?draw=3 (accessed June 2020)
[75] University of Oxford. Recruitment of adult patients to the Dexamethasone arm of the RECOVERY Trial is now closed. 2020. Available from https://www.recoverytrial.net/for-site-staff (accessed June 2020)
[76] British National Formulary. Dexamethasone. 2020. Available at: https://bnf.nice.org.uk/drug/dexamethasone.html (accessed June 2020)
[77] Medicines complete. Drugs in Pregnancy and Lactation. 2017. Available at: https://www.medicinescomplete.com/#/browse/dpl (accessed June 2020)
[78] Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem 2007;282(28):20047–20051. doi: 10.1074/jbc.R700004200
[79] Gralinski LE & Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol 2015;235(2):185–195. doi: 10.1002/path.4454
[80] Arabi Y, Shalhoub S, Mandourah Y. Al-Hameed et al. Ribavirin and interferon therapy for critically ill patients with Middle East Respiratory Syndrome: a multicenter observational study. Clin Infect Dis 2020;70(9):1837–1844. doi: 10.1093/cid/ciz544
[81] Al Ghamdi M, Alghamdi KM, Ghandoora Y et al. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis 2016;16:174. doi: 10.1186/s12879-016-1492-4
[82] Chan JF, Yao Y, Yeung M et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common Marmoset. J Infect Dis 2015; 212(12):1904–1913. doi: 10.1093/infdis/jiv392
[83] Scagnolari C, Vicenzi E, Bellomi F et al. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther 2004;9(6):1003-1011. PMID: 15651759
[84] EU Clinical Trials Register. 2020. Available at: https://www.clinicaltrialsregister.eu/ (accessed June 2020)
[85] US National Library of Medicine. 2020. Available at: https://clinicaltrials.gov/ (accessed June 2020)
[86] EU Clinical Trials Register. A randomised double-blind placebo-controlled trial to determine the safety and efficacy of inhaled SNG001. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04385095#contacts (accessed June 2020)
[87] University Medical Center Utrecht. Randomized, Embedded, Multifactorial, Adaptive Platform trial for Community-Acquired Pneumonia (REMAP-CAP). 2019. Available at: https://www.remapcap.org/ (accessed June 2020)
[88] Keating GM. Peginterferon-α-2a (40 kD): a review of its use in chronic hepatitis B. Drugs 2009;69:2633–2660. doi: 10.2165/11203660-000000000-00000
[89] Antonelli G, Scagnolari C, Vicenzi E et al. Treatment of SARS with human interferons. Lancet 2003;362 (9390):1158. doi: 10.1016/S0140-6736(03)14482-0
[90] National Institute for Health and Care Excellence. Beta interferons and glatiramer acetate for treating multiple sclerosis. Technology appraisal guidance [TA527]. 2018. Available at: https://www.nice.org.uk/guidance/ta527 (accessed June 2020)
[91] World Health Organization. Coronavirus disease (COVID-19) pandemic. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=EAIaIQobChMIwOeUtr_U6QIV1IBQBh2nhADpEAAYASAAEgJcbfD_BwE (accessed June 2020)
[92] Stewart K. Community management of pneumonia and suspected COVID-19. 2020. Pharm J 2020;304(7937):336–339. doi: 10.1211/PJ.2020.20207964
[93] Medicines and Healthcare products Regulatory Agency. MHRA issues a scientific opinion for the first medicine to treat COVID-19 in the UK. 2020. Available at: https://www.gov.uk/government/news/mhra-supports-the-use-of-remdesivir-as-the-first-medicine-to-treat-covid-19-in-the-uk (accessed June 2020)